AT-hook peptides bind the major and minor groove of AT-rich DNA duplexes
- PMID: 35212375
- PMCID: PMC8934665
- DOI: 10.1093/nar/gkac115
AT-hook peptides bind the major and minor groove of AT-rich DNA duplexes
Abstract
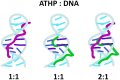
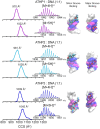
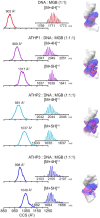
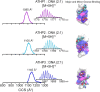
The mammalian high mobility group protein AT-hook 2 (HMGA2) houses three motifs that preferentially bind short stretches of AT-rich DNA regions. These DNA binding motifs, known as 'AT-hooks', are traditionally characterized as being unstructured. Upon binding to AT-rich DNA, they form ordered assemblies. It is this disordered-to-ordered transition that has implicated HMGA2 as a protein actively involved in many biological processes, with abnormal HMGA expression linked to a variety of health problems including diabetes, obesity, and oncogenesis. In the current work, the solution binding dynamics of the three 'AT-hook' peptides (ATHPs) with AT-rich DNA hairpin substrates were studied using DNA UV melting studies, fluorescence spectroscopy, native ion mobility spectrometry-mass spectrometry (IMS-MS), solution isothermal titration calorimetry (ITC) and molecular modeling. Results showed that the ATHPs bind to the DNA to form a single, 1:1 and 2:1, 'key-locked' conformational ensemble. The molecular models showed that 1:1 and 2:1 complex formation is driven by the capacity of the ATHPs to bind to the minor and major grooves of the AT-rich DNA oligomers. Complementary solution ITC results confirmed that the 2:1 stoichiometry of ATHP: DNA is originated under native conditions in solution.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


Similar articles
-
Crystal structure of the HMGA AT-hook 1 domain bound to the minor groove of AT-rich DNA and inhibition by antikinetoplastid drugs.Sci Rep. 2024 Oct 30;14(1):26173. doi: 10.1038/s41598-024-77522-3. Sci Rep. 2024. PMID: 39478017 Free PMC article.
-
Exploring the Conformational and Binding Dynamics of HMGA2·DNA Complexes Using Trapped Ion Mobility Spectrometry-Mass Spectrometry.J Am Soc Mass Spectrom. 2022 Jul 6;33(7):1103-1112. doi: 10.1021/jasms.2c00101. Epub 2022 Jun 10. J Am Soc Mass Spectrom. 2022. PMID: 35687119 Free PMC article.
-
Peptide Sequence Influence on the Conformational Dynamics and DNA binding of the Intrinsically Disordered AT-Hook 3 Peptide.Sci Rep. 2018 Jul 17;8(1):10783. doi: 10.1038/s41598-018-28956-z. Sci Rep. 2018. PMID: 30018295 Free PMC article.
-
The Mammalian High Mobility Group Protein AT-Hook 2 (HMGA2): Biochemical and Biophysical Properties, and Its Association with Adipogenesis.Int J Mol Sci. 2020 May 25;21(10):3710. doi: 10.3390/ijms21103710. Int J Mol Sci. 2020. PMID: 32466162 Free PMC article. Review.
-
HMGI/Y proteins: flexible regulators of transcription and chromatin structure.Biochim Biophys Acta. 2001 May 28;1519(1-2):13-29. doi: 10.1016/s0167-4781(01)00215-9. Biochim Biophys Acta. 2001. PMID: 11406267 Review.
Cited by
-
Crystal structure of the HMGA AT-hook 1 domain bound to the minor groove of AT-rich DNA and inhibition by antikinetoplastid drugs.Sci Rep. 2024 Oct 30;14(1):26173. doi: 10.1038/s41598-024-77522-3. Sci Rep. 2024. PMID: 39478017 Free PMC article.
-
An AT-hook transcription factor promotes transcription of histone, spliced-leader, and piRNA clusters.Nucleic Acids Res. 2025 Feb 8;53(4):gkaf079. doi: 10.1093/nar/gkaf079. Nucleic Acids Res. 2025. PMID: 39945323 Free PMC article.
-
Exploring the Conformations and Binding Location of HMGA2·DNA Complexes Using Ion Mobility Spectrometry and 193 nm Ultraviolet Photodissociation Mass Spectrometry.J Am Soc Mass Spectrom. 2022 Jul 6;33(7):1092-1102. doi: 10.1021/jasms.2c00083. Epub 2022 Jun 10. J Am Soc Mass Spectrom. 2022. PMID: 35687872 Free PMC article.
-
Exploring the Conformational and Binding Dynamics of HMGA2·DNA Complexes Using Trapped Ion Mobility Spectrometry-Mass Spectrometry.J Am Soc Mass Spectrom. 2022 Jul 6;33(7):1103-1112. doi: 10.1021/jasms.2c00101. Epub 2022 Jun 10. J Am Soc Mass Spectrom. 2022. PMID: 35687119 Free PMC article.
-
Inhibition of HMGA2 binding to AT-rich DNA by its negatively charged C-terminus.Nucleic Acids Res. 2025 Jan 24;53(3):gkaf035. doi: 10.1093/nar/gkaf035. Nucleic Acids Res. 2025. PMID: 39873271 Free PMC article.
References
-
- Bustin M. Revised nomenclature for high mobility group (HMG) chromosomal proteins. Trends Biochem. Sci. 2001; 26:152–153. - PubMed
-
- Reeves R., Nissen M.S.. The A.T-DNA-binding domain of mammalian high mobility group i chromosomal proteins. A novel peptide motif for recognizing DNA structure. J. Biol. Chem. 1990; 265:8573–8582. - PubMed
-
- Cui T., Leng F.. Specific recognition of AT-rich DNA sequences by the mammalian high mobility group protein AT-hook 2: a SELEX study. Biochemistry. 2007; 46:13059–13066. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

