Biodegradable Elastomers and Gels for Elastic Electronics
- PMID: 35212474
- PMCID: PMC9069371
- DOI: 10.1002/advs.202105146
Biodegradable Elastomers and Gels for Elastic Electronics
Abstract
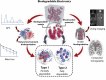
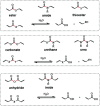
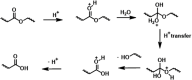
Biodegradable electronics are considered as an important bio-friendly solution for electronic waste (e-waste) management, sustainable development, and emerging implantable devices. Elastic electronics with higher imitative mechanical characteristics of human tissues, have become crucial for human-related applications. The convergence of biodegradability and elasticity has emerged a new paradigm of next-generation electronics especially for wearable and implantable electronics. The corresponding biodegradable elastic materials are recognized as a key to drive this field toward the practical applications. The review first clarifies the relevant concepts including biodegradable and elastic electronics along with their general design principles. Subsequently, the crucial mechanisms of the degradation in polymeric materials are discussed in depth. The diverse types of biodegradable elastomers and gels for electronics are then summarized. Their molecular design, modification, processing, and device fabrication especially the structure-properties relationship as well as recent advanced are reviewed in detail. Finally, the current challenges and the future directions are proposed. The critical insights of biodegradability and elastic characteristics in the elastomers and gel allows them to be tailored and designed more effectively for electronic applications.
Keywords: bio-friendly; biodegradable; elastic electronics; elastomers; gels; implantable electronics; wearable electronics.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




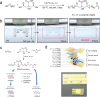



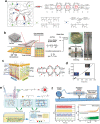

References
-
- Kim D.‐H., Lu N., Ma R., Kim Y.‐S., Kim R.‐H., Wang S., Wu J., Won S. M., Tao H., Islam A., Yu K. J., Kim T.‐i., Chowdhury R., Ying M., Xu L., Li M., Chung H.‐J., Keum H., McCormick M., Liu P., Zhang Y.‐W., Omenetto F. G., Huang Y., Coleman T., Rogers J. A., Science 2011, 333, 838. - PubMed
-
- Hammock M. L., Chortos A., Tee B. C.‐K., Tok J. B.‐H., Bao Z., Adv. Mater. 2013, 25, 5997. - PubMed
-
- Kaltenbrunner M., Sekitani T., Reeder J., Yokota T., Kuribara K., Tokuhara T., Drack M., Schwödiauer R., Graz I., Bauer‐Gogonea S., Bauer S., Someya T., Nature 2013, 499, 458. - PubMed
-
- Wang W., Yu A., Zhai J., Wang Z., Adv. Fiber Mater. 2021, 3, 394.
-
- Meng W., Nie M., Liu Z., Zhou J., Adv. Fiber Mater. 2021, 3, 149.
Publication types
MeSH terms
Substances
Grants and funding
- 2021YFC2101804/National Key Research and Development Program of China
- 2021YFC2101802/National Key Research and Development Program of China
- 52173117/National Natural Science Foundation of China
- 21991123/National Natural Science Foundation of China
- 20ZR1402500/Natural Science Foundation of Shanghai
- 20520741000/Belt & Road Young Scientist Exchanges Project of Science and Technology Commission Foundation of Shanghai
- 18520750400/Donghua University
- 20DZ2254900/Science and Technology Commission of Shanghai
- Fundamental Research Funds for the Central Universities
- LZA2019001/DHU Distinguished Young Professor Program
- 2019B10068/Ningbo 2025 Science and Technology Major Project
LinkOut - more resources
Full Text Sources
