Novel nitrite reductase domain structure suggests a chimeric denitrification repertoire in the phylum Chloroflexi
- PMID: 35212484
- PMCID: PMC8756737
- DOI: 10.1002/mbo3.1258
Novel nitrite reductase domain structure suggests a chimeric denitrification repertoire in the phylum Chloroflexi
Abstract
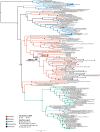
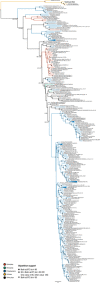
Denitrification plays a central role in the global nitrogen cycle, reducing and removing nitrogen from marine and terrestrial ecosystems. The flux of nitrogen species through this pathway has a widespread impact, affecting ecological carrying capacity, agriculture, and climate. Nitrite reductase (Nir) and nitric oxide reductase (NOR) are the two central enzymes in this pathway. Here we present a previously unreported Nir domain architecture in members of phylum Chloroflexi. Phylogenetic analyses of protein domains within Nir indicate that an ancestral horizontal transfer and fusion event produced this chimeric domain architecture. We also identify an expanded genomic diversity of a rarely reported NOR subtype, eNOR. Together, these results suggest a greater diversity of denitrification enzyme arrangements exist than have been previously reported.
Keywords: Chloroflexi; cytochrome; denitrification; nitric-oxide reductase; nitrite reductase; phylogeny.
© 2021 The Authors. MicrobiologyOpen published by John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures












References
-
- Agarwala, R. , Barrett, T. , Beck, J. , Benson, D. A. , Bollin, C. , Bolton, E. , Bourexis, D. , Brister, J. R. , Bryant, S. H. , Canese, K. , Cavanaugh, M. , Charowhas, C. , Clark, K. , Dondoshansky, I. , Feolo, M. , Fitzpatrick, L. , Funk, K. , Geer, L. Y. , Gorelenkov, V. , … Zbicz, K. (2018). Database resources of the National Center for Biotechnology Information. Nucleic Acids Research, 46, D8–D13. 10.1093/nar/gkx1095 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

