Glymphatic system clears extracellular tau and protects from tau aggregation and neurodegeneration
- PMID: 35212707
- PMCID: PMC8932543
- DOI: 10.1084/jem.20211275
Glymphatic system clears extracellular tau and protects from tau aggregation and neurodegeneration
Abstract
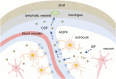
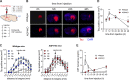
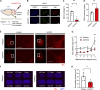
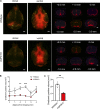
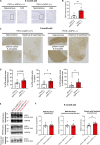
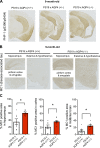
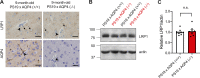
Accumulation of tau has been implicated in various neurodegenerative diseases termed tauopathies. Tau is a microtubule-associated protein but is also actively released into the extracellular fluids including brain interstitial fluid and cerebrospinal fluid (CSF). However, it remains elusive whether clearance of extracellular tau impacts tau-associated neurodegeneration. Here, we show that aquaporin-4 (AQP4), a major driver of the glymphatic clearance system, facilitates the elimination of extracellular tau from the brain to CSF and subsequently to deep cervical lymph nodes. Strikingly, deletion of AQP4 not only elevated tau in CSF but also markedly exacerbated phosphorylated tau deposition and the associated neurodegeneration in the brains of transgenic mice expressing P301S mutant tau. The current study identified the clearance pathway of extracellular tau in the central nervous system, suggesting that glymphatic clearance of extracellular tau is a novel regulatory mechanism whose impairment contributes to tau aggregation and neurodegeneration.
© 2022 Ishida et al.
Conflict of interest statement
Disclosures: K. Yamada reported “part of the study was supported by a collaborative grant from NIPRO Co.” No other disclosures were reported.
Figures

References
-
- Abe, Y., Ikegawa N., Yoshida K., Muramatsu K., Hattori S., Kawai K., Murakami M., Tanaka T., Goda W., Goto M., et al. . 2020. Behavioral and electrophysiological evidence for a neuroprotective role of aquaporin-4 in the 5xFAD transgenic mice model. Acta Neuropathol. Commun. 8:67. 10.1186/s40478-020-00936-3 - DOI - PMC - PubMed
-
- Barthélemy, N.R., Li Y., Joseph-Mathurin N., Gordon B.A., Hassenstab J., Benzinger T.L.S., Buckles V., Fagan A.M., Perrin R.J., Goate A.M., et al. 2020b. A soluble phosphorylated tau signature links tau, amyloid and the evolution of stages of dominantly inherited Alzheimer’s disease. Nat. Med. 26:398–407. 10.1038/s41591-020-0781-z - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

