Spatial Dissociation of Subretinal Drusenoid Deposits and Impaired Scotopic and Mesopic Sensitivity in AMD
- PMID: 35212721
- PMCID: PMC8883144
- DOI: 10.1167/iovs.63.2.32
Spatial Dissociation of Subretinal Drusenoid Deposits and Impaired Scotopic and Mesopic Sensitivity in AMD
Abstract
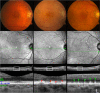
Purpose: Subretinal drusenoid deposits (SDD) first appear in the rod-rich perifovea and can extend to the cone-rich fovea. To refine the spatial relationship of visual dysfunction with SDD burden, we determined the topography of mesopic and scotopic light sensitivity in participants with non-neovascular AMD with and without SDD.
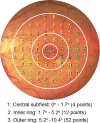
Methods: Thirty-three subjects were classified into three groups: normal (n = 9), AMD-Drusen (with drusen and without SDD; n = 12), and AMD-SDD (predominantly SDD; n = 12). Mesopic and scotopic microperimetry were performed using 68 targets within the Early Treatment Diabetic Retinopathy Study grid, including points at 1.7° from the foveal center (rod:cone ratio, 0.35). Age-adjusted linear regression was used to compare mesopic and scotopic light sensitivities across groups.
Results: Across the entire Early Treatment Diabetic Retinopathy Study grid and within individual subfields, the three groups differed significantly for mesopic and scotopic light sensitivities (all P < 0.05). The AMD-SDD group exhibited significantly decreased mesopic and scotopic sensitivity versus both the normal and the AMD-Drusen groups (all P < 0.05), while AMD-Drusen and normal eyes did not significantly differ (all P > 0.05). The lowest relative sensitivities were recorded for scotopic light levels, especially in the central subfield, in the AMD-SDD group.
Conclusions: SDD-associated decrements in rod-mediated vision can be detected close to the foveola, and these deficits are proportionately worse than functional loss in the rod-rich perifovea. This finding suggests that factors other than the previously hypothesized direct cytotoxicity to photoreceptors and local transport barrier limitations may negatively impact vision. Larger prospective studies are required to confirm these observations.
Conflict of interest statement
Disclosure:
Figures


Similar articles
-
Extent and Topography of Subretinal Drusenoid Deposits Associate With Rod-Mediated Vision in Aging and AMD: ALSTAR2 Baseline.Invest Ophthalmol Vis Sci. 2024 Aug 1;65(10):25. doi: 10.1167/iovs.65.10.25. Invest Ophthalmol Vis Sci. 2024. PMID: 39163034 Free PMC article.
-
Retinal Sensitivity Using Microperimetry in Age-Related Macular Degeneration in an Amish Population.Ophthalmic Surg Lasers Imaging Retina. 2019 Sep 1;50(9):e236-e241. doi: 10.3928/23258160-20190905-15. Ophthalmic Surg Lasers Imaging Retina. 2019. PMID: 31589764
-
Spatially Resolved Association of Structural Biomarkers on Retinal Function in Non-Exudative Age-Related Macular Degeneration Over 4 Years.Invest Ophthalmol Vis Sci. 2024 Apr 1;65(4):45. doi: 10.1167/iovs.65.4.45. Invest Ophthalmol Vis Sci. 2024. PMID: 38687492 Free PMC article.
-
Role of microperimetry in evaluating disease progression in age-related macular degeneration: a scoping review.Int Ophthalmol. 2022 Jun;42(6):1975-1986. doi: 10.1007/s10792-021-02170-9. Epub 2022 Jan 7. Int Ophthalmol. 2022. PMID: 34994874 Free PMC article.
-
Retinal sensitivity changes in early/intermediate AMD: a systematic review and meta-analysis of visual field testing under mesopic and scotopic lighting.Eye (Lond). 2024 Jul;38(10):1827-1835. doi: 10.1038/s41433-024-03033-0. Epub 2024 Mar 18. Eye (Lond). 2024. PMID: 38499857 Free PMC article.
Cited by
-
Choriocapillaris Impairment Is Associated With Delayed Rod-Mediated Dark Adaptation in Age-Related Macular Degeneration.Invest Ophthalmol Vis Sci. 2023 Sep 1;64(12):41. doi: 10.1167/iovs.64.12.41. Invest Ophthalmol Vis Sci. 2023. PMID: 37768273 Free PMC article.
-
Histology of Type 3 Macular Neovascularization and Microvascular Anomalies in Treated Age-Related Macular Degeneration: A Case Study.Ophthalmol Sci. 2023 Feb 10;3(3):100280. doi: 10.1016/j.xops.2023.100280. eCollection 2023 Sep. Ophthalmol Sci. 2023. PMID: 36970117 Free PMC article.
-
Progression Patterns in Foveal-Sparing Geographic Atrophy With Double-Layer Sign Due to Neovascularization or Basal Laminar Deposits.Invest Ophthalmol Vis Sci. 2025 Aug 1;66(11):16. doi: 10.1167/iovs.66.11.16. Invest Ophthalmol Vis Sci. 2025. PMID: 40767440 Free PMC article.
-
Photoreceptor Function and Structure in Retinal Areas With Intraretinal Hyperreflective Foci in Age-Related Macular Degeneration.Invest Ophthalmol Vis Sci. 2025 Feb 3;66(2):27. doi: 10.1167/iovs.66.2.27. Invest Ophthalmol Vis Sci. 2025. PMID: 39928312 Free PMC article.
-
Extent and Topography of Subretinal Drusenoid Deposits Associate With Rod-Mediated Vision in Aging and AMD: ALSTAR2 Baseline.Invest Ophthalmol Vis Sci. 2024 Aug 1;65(10):25. doi: 10.1167/iovs.65.10.25. Invest Ophthalmol Vis Sci. 2024. PMID: 39163034 Free PMC article.
References
-
- Spaide RF. Improving the age-related macular degeneration construct: a new classification system. Retina . 2018; 38(5): 891–899. - PubMed
-
- Curcio CA, Millican CL, Allen KA, Kalina RE.. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Invest Ophthalmol Vis Sci . 1993; 34(12): 3278–3296. - PubMed
-
- Curcio CA, Medeiros NE, Millican CL.. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci . 1996; 37(7): 1236–1249. - PubMed
-
- Adler R, Curcio C, Hicks D, Price D, Wong F.. Cell death in age-related macular degeneration. Mol Vis . 1999; 5: 31. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

