Gut Microbiota Regulate Pancreatic Growth, Exocrine Function, and Gut Hormones
- PMID: 35212729
- PMCID: PMC9044125
- DOI: 10.2337/db21-0382
Gut Microbiota Regulate Pancreatic Growth, Exocrine Function, and Gut Hormones
Abstract
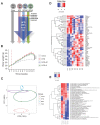
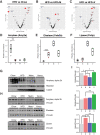
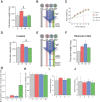
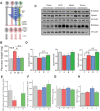
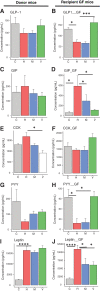
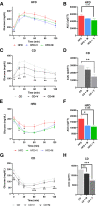
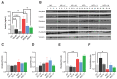
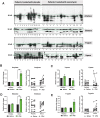
Growing evidence indicates an important link between gut microbiota, obesity, and metabolic syndrome. Alterations in exocrine pancreatic function are also widely present in patients with diabetes and obesity. To examine this interaction, C57BL/6J mice were fed a chow diet, a high-fat diet (HFD), or an HFD plus oral vancomycin or metronidazole to modify the gut microbiome. HFD alone leads to a 40% increase in pancreas weight, decreased glucagon-like peptide 1 and peptide YY levels, and increased glucose-dependent insulinotropic peptide in the plasma. Quantitative proteomics identified 138 host proteins in fecal samples of these mice, of which 32 were significantly changed by the HFD. The most significant of these were the pancreatic enzymes. These changes in amylase and elastase were reversed by antibiotic treatment. These alterations could be reproduced by transferring gut microbiota from donor C57BL/6J mice to germ-free mice. By contrast, antibiotics had no effect on pancreatic size or exocrine function in C57BL/6J mice fed the chow diet. Further, 1 week vancomycin administration significantly increased amylase and elastase levels in obese men with prediabetes. Thus, the alterations in gut microbiota in obesity can alter pancreatic growth, exocrine function, and gut endocrine function and may contribute to the alterations observed in patients with obesity and diabetes.
© 2022 by the American Diabetes Association.
Figures
Similar articles
-
Short Term High Fat Diet Induces Obesity-Enhancing Changes in Mouse Gut Microbiota That are Partially Reversed by Cessation of the High Fat Diet.Lipids. 2017 Jun;52(6):499-511. doi: 10.1007/s11745-017-4253-2. Epub 2017 Apr 20. Lipids. 2017. PMID: 28429150
-
Phlorizin ameliorates obesity-associated endotoxemia and insulin resistance in high-fat diet-fed mice by targeting the gut microbiota and intestinal barrier integrity.Gut Microbes. 2020 Nov 9;12(1):1-18. doi: 10.1080/19490976.2020.1842990. Gut Microbes. 2020. PMID: 33222603 Free PMC article.
-
Suppression of High-Fat Diet-Induced Obesity by Platycodon Grandiflorus in Mice Is Linked to Changes in the Gut Microbiota.J Nutr. 2020 Sep 1;150(9):2364-2374. doi: 10.1093/jn/nxaa159. J Nutr. 2020. PMID: 32510156
-
Improvement in glucose tolerance and insulin sensitivity by probiotic strains of Indian gut origin in high-fat diet-fed C57BL/6J mice.Eur J Nutr. 2018 Feb;57(1):279-295. doi: 10.1007/s00394-016-1317-7. Epub 2016 Oct 18. Eur J Nutr. 2018. PMID: 27757592
-
The metabolic and vascular protective effects of olive (Olea europaea L.) leaf extract in diet-induced obesity in mice are related to the amelioration of gut microbiota dysbiosis and to its immunomodulatory properties.Pharmacol Res. 2019 Dec;150:104487. doi: 10.1016/j.phrs.2019.104487. Epub 2019 Oct 11. Pharmacol Res. 2019. PMID: 31610229
Cited by
-
Exploring a novel therapeutic strategy: the interplay between gut microbiota and high-fat diet in the pathogenesis of metabolic disorders.Front Nutr. 2023 Dec 15;10:1291853. doi: 10.3389/fnut.2023.1291853. eCollection 2023. Front Nutr. 2023. PMID: 38192650 Free PMC article. Review.
-
The association between exocrine pancreatic insufficiency and changes in gut microbiota: a narrative review.Med Pharm Rep. 2025 Jan;98(1):5-12. doi: 10.15386/mpr-2638. Epub 2025 Jan 31. Med Pharm Rep. 2025. PMID: 39949904 Free PMC article. Review.
-
Murine gut microbiota dysbiosis via enteric infection modulates the foreign body response to a distal biomaterial implant.Proc Natl Acad Sci U S A. 2025 May 20;122(20):e2422169122. doi: 10.1073/pnas.2422169122. Epub 2025 May 12. Proc Natl Acad Sci U S A. 2025. PMID: 40354538
-
Type 2 diabetes, gut microbiome, and systems biology: A novel perspective for a new era.Gut Microbes. 2022 Jan-Dec;14(1):2111952. doi: 10.1080/19490976.2022.2111952. Gut Microbes. 2022. PMID: 36004400 Free PMC article. Review.
-
Innovative treatments for obesity and NAFLD: A bibliometric study on antioxidants, herbs, phytochemicals, and natural compounds.Heliyon. 2024 Aug 8;10(16):e35498. doi: 10.1016/j.heliyon.2024.e35498. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39220898 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

