Gestational Cd Exposure in the CD-1 Mouse Sex-Specifically Disrupts Essential Metal Ion Homeostasis
- PMID: 35212737
- PMCID: PMC9154225
- DOI: 10.1093/toxsci/kfac027
Gestational Cd Exposure in the CD-1 Mouse Sex-Specifically Disrupts Essential Metal Ion Homeostasis
Abstract
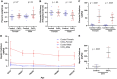
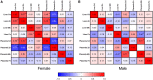
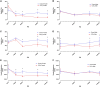
In CD-1 mice, gestational-only exposure to cadmium (Cd) causes female-specific hepatic insulin resistance, metabolic disruption, and obesity. To evaluate whether sex differences in uptake and changes in essential metal concentrations contribute to metabolic outcomes, placental and liver Cd and essential metal concentrations were quantified in male and female offspring perinatally exposed to 500 ppb CdCl2. Exposure resulted in increased maternal liver Cd+2 concentrations (364 µg/kg) similar to concentrations found in non-occupationally exposed human liver. At gestational day (GD) 18, placental Cd and manganese concentrations were significantly increased in exposed males and females, and zinc was significantly decreased in females. Placental efficiency was significantly decreased in GD18-exposed males. Increases in hepatic Cd concentrations and a transient prenatal increase in zinc were observed in exposed female liver. Fetal and adult liver iron concentrations were decreased in both sexes, and decreases in hepatic zinc, iron, and manganese were observed in exposed females. Analysis of GD18 placental and liver metallothionein mRNA expression revealed significant Cd-induced upregulation of placental metallothionein in both sexes, and a significant decrease in fetal hepatic metallothionein in exposed females. In placenta, expression of metal ion transporters responsible for metal ion uptake was increased in exposed females. In liver of exposed adult female offspring, expression of the divalent cation importer (Slc39a14/Zip14) decreased, whereas expression of the primary exporter (Slc30a10/ZnT10) increased. These findings demonstrate that Cd can preferentially cross the female placenta, accumulate in the liver, and cause lifelong dysregulation of metal ion concentrations associated with metabolic disruption.
Keywords: epigenetic; essential metals; gestation; metallothionein; placenta.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Andrews G. K. (1990). Regulation of metallothionein gene expression. Prog. Food Nutr. Sci. 14, 193–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

