Natural compounds as safe therapeutic options for ulcerative colitis
- PMID: 35212849
- PMCID: PMC8948151
- DOI: 10.1007/s10787-022-00931-1
Natural compounds as safe therapeutic options for ulcerative colitis
Abstract
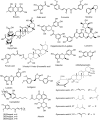
Ulcerative colitis (UC) is a chronic inflammatory bowel disease of unknown etiology. Several conventional treatments for UC such as corticosteroids, immunosuppressive agents, tumor necrosis factor antagonist, integrin blockers, and interleukin antagonist, and salicylates are available but are associated with the various limitations and side-effects. None of the above treatments helps to achieve the ultimate goal of the therapy, i.e., maintenance of remission in the long-term. Natural remedies for the treatment of UC show comparatively less side effects as compared to conventional approaches, and affordable. The current review presents details on the role of herbal drugs in the treatment and cure of UC. Google, PubMed, Web of Science, and Scopus portals have been searched for potentially relevant literature to get the latest developments and updated information related to use of natural drugs in the treatment of UC. Natural products have been used over centuries to treat UC. Some of the essential herbal constituents exhibiting antiulcerogenic activity include gymnemic acid (Gymnema sylvestre), shagoal (Zingiber officinale), catechin (Camellia sinensis), curcumin (Curcuma longa), arctigenin (Arctium lappa), and boswellic acid (Boswellia serrata). Although many plant-derived products have been recommended for UC, further research to understand the exact molecular mechanism is still warranted to establish their usefulness clinically.
Keywords: Anti-ulcerogenic activity; Herbal constituents; Inflammatory bowel disease; Ulcerative colitis.
© 2022. The Author(s).
Conflict of interest statement
Authors confirm that there are no known conflicts of interest associated with this work.
Figures



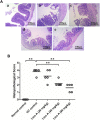
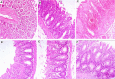

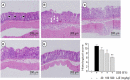


References
-
- Al-Snafi A. The pharmacological importance and chemical constituents of Arctiumlappa. A review. Int J Pharm Res Scholar. 2014;3:663–670.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
