Characteristics of New Biologic Users Among the Moderate-to-Severe Psoriasis Population-Retrospective Cohort Study Leveraging the Modernizing Medicine Data Services Database
- PMID: 35212934
- PMCID: PMC8941060
- DOI: 10.1007/s13555-022-00691-4
Characteristics of New Biologic Users Among the Moderate-to-Severe Psoriasis Population-Retrospective Cohort Study Leveraging the Modernizing Medicine Data Services Database
Abstract
Introduction: Biologics have expanded the treatment options in the management of patients with moderate-to-severe psoriasis. The objective of this study was to describe patient characteristics and previous treatments in psoriasis patients newly treated with guselkumab, secukinumab, or ixekizumab.
Methods: This retrospective study included patients ≥ 18 years old with psoriasis in the USA who were newly treated with guselkumab, secukinumab, or ixekizumab between 1 July 2017 and 31 March 2019 in the Modernizing Medicine Data Services database (MMDS). Patients were indexed on their first prescription or injection record of guselkumab, secukinumab, or ixekizumab, and three mutually exclusive cohorts were created. Patients were required to have evidence of moderate-to-severe psoriasis, defined as Physician Global Assessment (PGA) score of 3 or 4, or body surface area (BSA) ≥ 10% on index date or within 12 months before index. Baseline characteristics, including treatment history, were reported for each cohort.
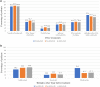
Results: The study population included 461 guselkumab, 619 secukinumab, and 375 ixekizumab patients. The median age across cohorts was 51-52 years. Median baseline BSA ranged from 15% to 20%; 16.1-29.3% of patients had a PGA of 4 and over half of patients were obese prior to index. Approximately 40% of patients had comorbid cardiovascular disease and 20.8-24.2% of patients had a psychiatric disorder. About half of patients in each cohort had prior biologic use, of which adalimumab was most common (28.2-34.9%) across the cohorts.
Conclusion: This real-world study describes the characteristics of patients with moderate-to-severe psoriasis receiving biologic treatments.
Keywords: Biologics; Body surface area; Electronic medical record; Guselkumab; Ixekizumab; Modernizing medicine data services; Physician global assessment; Psoriasis; Real-world; Secukinumab.
© 2022. The Author(s).
Figures
References
LinkOut - more resources
Full Text Sources