Gymnemic Acid Ameliorates Pancreatic β-Cell Dysfunction by Modulating Pdx1 Expression: A Possible Strategy for β-Cell Regeneration
- PMID: 35212973
- PMCID: PMC9130387
- DOI: 10.1007/s13770-022-00435-7
Gymnemic Acid Ameliorates Pancreatic β-Cell Dysfunction by Modulating Pdx1 Expression: A Possible Strategy for β-Cell Regeneration
Abstract
Background: Endogenous pancreatic β-cell regeneration is a promising therapeutic approach for enhancing β-cell function and neogenesis in diabetes. Various findings have reported that regeneration might occur via stimulating β-cell proliferation, neogenesis, or conversion from other pancreatic cells to β-like cells. Although the current scenario illustrates numerous therapeutic strategies and approaches that concern endogenous β-cell regeneration, all of them have not been successful to a greater extent because of cost effectiveness, availability of suitable donors and rejection in case of transplantation, or lack of scientific evidence for many phytochemicals derived from plants that have been employed in traditional medicine. Therefore, the present study aims to investigate the effect of gymnemic acid (GA) on β-cell regeneration in streptozotocin-induced type 1 diabetic rats and high glucose exposed RIN5-F cells.
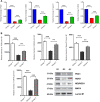
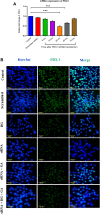
Methods: The study involves histopathological and immunohistochemical analysis to examine the islet's architecture. Quantitative polymerase chain reaction (qPCR) and/or immunoblot were employed to quantify the β-cell regeneration markers and cell cycle proliferative markers.
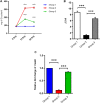
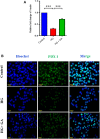
Results: The immunoexpression of E-cadherin, β-catenin, and phosphoinositide 3-kinases/protein kinase B were significantly increased in GA-treated diabetic rats. On the other hand, treatment with GA upregulated the pancreatic regenerative transcription factor viz. pancreatic duodenal homeobox 1, Neurogenin 3, MafA, NeuroD1, and β-cells proliferative markers such as CDK4, and Cyclin D1, with a simultaneous downregulation of the forkhead box O, glycogen synthase kinase-3, and p21cip1 in diabetic treated rats. Adding to this, we noticed increased nuclear localization of Pdx1 in GA treated high glucose exposed RIN5-F cells.
Conclusion: Our results suggested that GA acts as a potential therapeutic candidate for endogenous β-cell regeneration in treating type 1 diabetes.
Keywords: Diabetes; Gymnemic acid; Islet architecture; β-Cell proliferation; β-Cell regeneration.
© 2022. The Korean Tissue Engineering and Regenerative Medicine Society.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Richardson T, Kerr D. Skin-related complications of insulin therapy: epidemiology and emerging management strategies. Am J Clin Dermatol. 2003;4:661–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
