Reliever-Triggered Inhaled Glucocorticoid in Black and Latinx Adults with Asthma
- PMID: 35213105
- PMCID: PMC10367430
- DOI: 10.1056/NEJMoa2118813
Reliever-Triggered Inhaled Glucocorticoid in Black and Latinx Adults with Asthma
Abstract
Background: Black and Latinx patients bear a disproportionate burden of asthma. Efforts to reduce the disproportionate morbidity have been mostly unsuccessful, and guideline recommendations have not been based on studies in these populations.
Methods: In this pragmatic, open-label trial, we randomly assigned Black and Latinx adults with moderate-to-severe asthma to use a patient-activated, reliever-triggered inhaled glucocorticoid strategy (beclomethasone dipropionate, 80 μg) plus usual care (intervention) or to continue usual care. Participants had one instructional visit followed by 15 monthly questionnaires. The primary end point was the annualized rate of severe asthma exacerbations. Secondary end points included monthly asthma control as measured with the Asthma Control Test (ACT; range, 5 [poor] to 25 [complete control]), quality of life as measured with the Asthma Symptom Utility Index (ASUI; range, 0 to 1, with lower scores indicating greater impairment), and participant-reported missed days of work, school, or usual activities. Safety was also assessed.
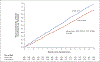
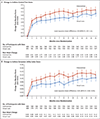
Results: Of 1201 adults (603 Black and 598 Latinx), 600 were assigned to the intervention group and 601 to the usual-care group. The annualized rate of severe asthma exacerbations was 0.69 (95% confidence interval [CI], 0.61 to 0.78) in the intervention group and 0.82 (95% CI, 0.73 to 0.92) in the usual-care group (hazard ratio, 0.85; 95% CI, 0.72 to 0.999; P = 0.048). ACT scores increased by 3.4 points (95% CI, 3.1 to 3.6) in the intervention group and by 2.5 points (95% CI, 2.3 to 2.8) in the usual-care group (difference, 0.9; 95% CI, 0.5 to 1.2); ASUI scores increased by 0.12 points (95% CI, 0.11 to 0.13) and 0.08 points (95% CI, 0.07 to 0.09), respectively (difference, 0.04; 95% CI, 0.02 to 0.05). The annualized rate of missed days was 13.4 in the intervention group and 16.8 in the usual-care group (rate ratio, 0.80; 95% CI, 0.67 to 0.95). Serious adverse events occurred in 12.2% of the participants, with an even distribution between the groups.
Conclusions: Among Black and Latinx adults with moderate-to-severe asthma, provision of an inhaled glucocorticoid and one-time instruction on its use, added to usual care, led to a lower rate of severe asthma exacerbations. (Funded by the Patient-Centered Outcomes Research Institute and others; PREPARE ClinicalTrials.gov number, NCT02995733.).
Copyright © 2022 Massachusetts Medical Society.
Figures

Comment in
-
Breaking the Skin Color Barriers for Asthma Medications - It's Not Black, Brown, or White.N Engl J Med. 2022 Apr 21;386(16):1574-1575. doi: 10.1056/NEJMe2201666. N Engl J Med. 2022. PMID: 35443113 No abstract available.
-
In Black and Latinx patients, as-needed inhaled glucocorticoids reduced asthma exacerbations at 15 mo.Ann Intern Med. 2022 Jun;175(6):JC68. doi: 10.7326/J22-0040. Epub 2022 Jun 7. Ann Intern Med. 2022. PMID: 35667061
-
The time to PREPARE is over; the time to improve diversity in asthma studies is now.J Allergy Clin Immunol. 2022 Nov;150(5):1057-1058. doi: 10.1016/j.jaci.2022.09.014. Epub 2022 Sep 22. J Allergy Clin Immunol. 2022. PMID: 36154847 Free PMC article. No abstract available.
References
-
- Centers for Disease Control and Prevention. Most recent national asthma data (https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm).
-
- Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008–2013. Ann Am Thorac Soc 2018;15:348–56. - PubMed
-
- Sullivan SD, Wenzel SE, Bresnahan BW, et al. Association of control and risk of severe asthma-related events in severe or difficult-to-treat asthma patients. Allergy 2007;62:655–60. - PubMed
-
- Leong AB, Ramsey CD, Celedón JC. The challenge of asthma in minority populations. Clin Rev Allergy Immunol 2012;43:156–83. - PubMed
-
- Gold DR, Wright R. Population disparities in asthma. Annu Rev Public Health 2005;26:89–113. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
