Large-scale implementation of rapid antigen testing system for COVID-19 in workplaces
- PMID: 35213224
- PMCID: PMC8880770
- DOI: 10.1126/sciadv.abm3608
Large-scale implementation of rapid antigen testing system for COVID-19 in workplaces
Abstract
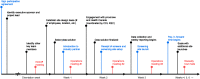
The transmission of coronavirus disease 2019 (COVID-19) in workplaces has been a persistent issue throughout the pandemic. In response, a not-for-profit initiative emerged to mitigate COVID-19 workplace transmission in Canada. We report the process for establishing a workplace frequent rapid antigen test (RAT) program. The screening program identified 473 asymptomatic individuals who tested positive on the RAT and confirmed positive by a nasopharyngeal polymerase chain reaction (PCR) diagnostic test. One in 4300 RATs was presumptive positive but later tested PCR negative, and thus, false positives did not meaningfully disrupt workplace operations. Most employers rated the program highly and felt strongly that the program contributed to workplace and community safety. The findings describe a sustained and scalable implementation plan for establishing a frequent workplace testing program. High-frequency testing programs offer the potential to break chains of transmission and act as an extra layer of protection in a comprehensive public health response.
Figures


References
-
- Mina M. J., Andersen K. G., COVID-19 testing: One size does not fit all. Science 371, 126–127 (2021). - PubMed
-
- Brümmer L. E., Katzenschlager S., Gaeddert M., Erdmann C., Schmitz S., Bota M., Grilli M., Larmann J., Weigand M. A., Pollock N. R., Macé A., Carmona S., Ongarello S., Sacks J. A., Denkinger C. M., Accuracy of novel antigen rapid diagnostics for SARS-CoV-2: A living systematic review and meta-analysis. PLoS Med. 18, e1003735 (2021). - PMC - PubMed
-
- Siddiqui Z. K., Chaudhary M., Robinson M., McCall A. B., Peralta R., Esteve R., Callahan C. W., Manabe Y. C., Campbell J. D., Johnson K. J., Elhabashy M., Kantsiper M.; CONQUER COVID Consortium, Ficke J. R., Implementation and accuracy of BinaxNOW rapid antigen COVID-19 test in asymptomatic and symptomatic populations in a high-volume self-referred testing site. Microbiol. Spectr. 9, e01008-21 (2021). - PMC - PubMed
-
- J. Gans, The Pandemic Information Gap: The Brutal Economics of COVID-19 (MIT Press, 2020).
LinkOut - more resources
Full Text Sources

