Circadian rhythms modulate the effect of eccentric exercise on rat soleus muscles
- PMID: 35213577
- PMCID: PMC8880858
- DOI: 10.1371/journal.pone.0264171
Circadian rhythms modulate the effect of eccentric exercise on rat soleus muscles
Abstract
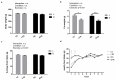
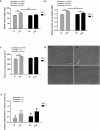
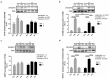
We investigated whether time-of-day dependent changes in the rat soleus (SOL) muscle size, after eccentric exercises, operate via the mechanistic target of rapamycin (mTOR) signaling pathway. For our first experiment, we assigned 9-week-old male Wistar rats randomly into four groups: light phase (zeitgeber time; ZT6) non-trained control, dark phase (ZT18) non-trained control, light phase-trained, and dark phase-trained. Trained animals performed 90 min of downhill running once every 3 d for 8 weeks. The second experiment involved dividing 9-week-old male Wistar rats to control and exercise groups. The latter were subjected to 15 min of downhill running at ZT6 and ZT18. The absolute (+12.8%) and relative (+9.4%) SOL muscle weights were higher in the light phase-trained group. p70S6K phosphorylation ratio was 42.6% higher in the SOL muscle of rats that had exercised only in light (non-trained ZT6). Collectively, the degree of muscle hypertrophy in SOL is time-of-day dependent, perhaps via the mTOR/p70S6K signaling.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Acute heat stress prior to downhill running may enhance skeletal muscle remodeling.Cell Stress Chaperones. 2012 Nov;17(6):693-705. doi: 10.1007/s12192-012-0343-5. Epub 2012 May 17. Cell Stress Chaperones. 2012. PMID: 22589083 Free PMC article.
-
Sex-specific differences in rat soleus muscle signaling pathway responses to a bout of horizontal and downhill running.J Physiol Biochem. 2019 Nov;75(4):585-595. doi: 10.1007/s13105-019-00712-5. Epub 2019 Nov 22. J Physiol Biochem. 2019. PMID: 31758515
-
The α₇β₁-integrin increases muscle hypertrophy following multiple bouts of eccentric exercise.J Appl Physiol (1985). 2011 Oct;111(4):1134-41. doi: 10.1152/japplphysiol.00081.2011. Epub 2011 Aug 4. J Appl Physiol (1985). 2011. PMID: 21817112
-
Circadian rhythm of intracellular protein synthesis signaling in rat cardiac and skeletal muscles.Biochem Biophys Rep. 2016 Dec 23;9:153-158. doi: 10.1016/j.bbrep.2016.12.005. eCollection 2017 Mar. Biochem Biophys Rep. 2016. PMID: 28956001 Free PMC article.
-
Contraction mode itself does not determine the level of mTORC1 activity in rat skeletal muscle.Physiol Rep. 2016 Oct;4(19):e12976. doi: 10.14814/phy2.12976. Physiol Rep. 2016. PMID: 27688433 Free PMC article.
Cited by
-
Time-of-day effect of high-intensity muscle contraction on mTOR signaling and protein synthesis in mice.Sci Rep. 2025 Jul 3;15(1):23702. doi: 10.1038/s41598-025-06709-z. Sci Rep. 2025. PMID: 40610492 Free PMC article.
-
Timed exercise stabilizes behavioral rhythms but not molecular programs in the brain's suprachiasmatic clock.iScience. 2023 Jan 18;26(2):106002. doi: 10.1016/j.isci.2023.106002. eCollection 2023 Feb 17. iScience. 2023. PMID: 36866044 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

