Human IL-2Rɑ subunit binding modulation of IL-2 through a decline in electrostatic interactions: A computational and experimental approach
- PMID: 35213635
- PMCID: PMC8880607
- DOI: 10.1371/journal.pone.0264353
Human IL-2Rɑ subunit binding modulation of IL-2 through a decline in electrostatic interactions: A computational and experimental approach
Abstract
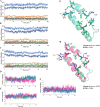
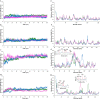
Although high-dose IL-2 has clear antitumor effects, severe side effects like severe toxicity and activation of Tregs by binding of IL-2 to high-affinity IL-2R, hypotension, and vascular leak syndrome limit its applications as a therapeutic antitumor agent. Here in this study, a rational computational approach was employed to develop and design novel triple-mutant IL-2 variants with the aim of improving IL-2-based immunotherapy. The affinity of the mutants towards IL-2Rα was further computed with the aid of molecular dynamic simulations and umbrella sampling techniques and the obtained results were compared to those of wild-type IL-2. In vitro experiments by flow cytometry showed that the anti-CD25 mAb was able to bind to PBMC cells even after mutant 2 preincubation, however, the binding strength of the mutant to α-subunit was less than of wtIL-2. Additionally, reduction of IL-2Rα subunit affinity did not significantly disturb IL-2/IL2Rβγc subunits interactions.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

