A preclinical model of patient-derived cerebrospinal fluid circulating tumor cells for experimental therapeutics in leptomeningeal disease from melanoma
- PMID: 35213727
- PMCID: PMC9527526
- DOI: 10.1093/neuonc/noac054
A preclinical model of patient-derived cerebrospinal fluid circulating tumor cells for experimental therapeutics in leptomeningeal disease from melanoma
Erratum in
-
Corrigendum to: A preclinical model of patient-derived cerebrospinal fluid circulating tumor cells for experimental therapeutics in leptomeningeal disease from melanoma.Neuro Oncol. 2022 Nov 2;24(11):2009. doi: 10.1093/neuonc/noac181. Neuro Oncol. 2022. PMID: 35904120 Free PMC article. No abstract available.
Abstract
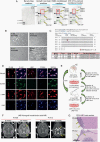
Background: Leptomeningeal disease (LMD) occurs as a late complication of several human cancers and has no rationally designed treatment options. A major barrier to developing effective therapies for LMD is the lack of cell-based or preclinical models that recapitulate human disease. Here, we describe the development of in vitro and in vivo cultures of patient-derived cerebrospinal fluid circulating tumor cells (PD-CSF-CTCs) from patients with melanoma as a preclinical model to identify exploitable vulnerabilities in melanoma LMD.
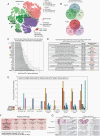
Methods: CSF-CTCs were collected from melanoma patients with melanoma-derived LMD and cultured ex vivo using human meningeal cell-conditioned media. Using immunoassays and RNA-sequencing analyses of PD-CSF-CTCs, molecular signaling pathways were examined and new therapeutic targets were tested for efficacy in PD-CSF-CTCs preclinical models.
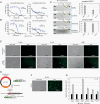
Results: PD-CSF-CTCs were successfully established both in vitro and in vivo. Global RNA analyses of PD-CSF-CTCs revealed several therapeutically tractable targets. These studies complimented our prior proteomic studies highlighting IGF1 signaling as a potential target in LMD. As a proof of concept, combining treatment of ceritinib and trametinib in vitro and in vivo demonstrated synergistic antitumor activity in PD-CSF-CTCs and BRAF inhibitor-resistant melanoma cells.
Conclusions: This study demonstrates that CSF-CTCs can be grown in vitro and in vivo from some melanoma patients with LMD and used as preclinical models. These models retained melanoma expression patterns and had signaling pathways that are therapeutically targetable. These novel models/reagents may be useful in developing rationally designed treatments for LMD.
Keywords: ceritinib; leptomeningeal disease (LMD); melanoma; patient-derived CSF-CTCs (PD-CSF-CTCs); single-cell RNA sequencing.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Figures

Comment in
-
Preclinical modeling in leptomeningeal disease: Starting at the foundation to tackle a difficult disease.Neuro Oncol. 2022 Oct 3;24(10):1687-1688. doi: 10.1093/neuonc/noac142. Neuro Oncol. 2022. PMID: 35751573 Free PMC article. No abstract available.
References
-
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–1546. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

