Patisiran treatment in patients with hereditary transthyretin-mediated amyloidosis with polyneuropathy after liver transplantation
- PMID: 35213769
- PMCID: PMC9310767
- DOI: 10.1111/ajt.17009
Patisiran treatment in patients with hereditary transthyretin-mediated amyloidosis with polyneuropathy after liver transplantation
Abstract
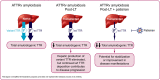
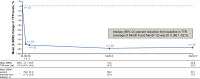
Hereditary transthyretin-mediated (hATTR) amyloidosis, or ATTRv amyloidosis, is a progressive disease, for which liver transplantation (LT) has been a long-standing treatment. However, disease progression continues post-LT. This Phase 3b, open-label trial evaluated efficacy and safety of patisiran in patients with ATTRv amyloidosis with polyneuropathy progression post-LT. Primary endpoint was median transthyretin (TTR) reduction from baseline. Twenty-three patients received patisiran for 12 months alongside immunosuppression regimens. Patisiran elicited a rapid, sustained TTR reduction (median reduction [Months 6 and 12 average], 91.0%; 95% CI: 86.1%-92.3%); improved neuropathy, quality of life, and autonomic symptoms from baseline to Month 12 (mean change [SEM], Neuropathy Impairment Score, -3.7 [2.7]; Norfolk Quality of Life-Diabetic Neuropathy questionnaire, -6.5 [4.9]; least-squares mean [SEM], Composite Autonomic Symptom Score-31, -5.0 [2.6]); and stabilized disability (Rasch-built Overall Disability Scale) and nutritional status (modified body mass index). Adverse events were mild or moderate; five patients experienced ≥1 serious adverse event. Most patients had normal liver function tests. One patient experienced transplant rejection consistent with inadequate immunosuppression, remained on patisiran, and completed the study. In conclusion, patisiran reduced serum TTR, was well tolerated, and improved or stabilized key disease impairment measures in patients with ATTRv amyloidosis with polyneuropathy progression post-LT (www.clinicaltrials.gov NCT03862807).
Keywords: clinical research/practice; clinical trial; liver allograft function/dysfunction; liver transplantation/hepatology; molecular biology: small interfering RNA; neurology; patient survival; pharmacology.
© 2022 The Authors. American Journal of Transplantation published by Wiley Periodicals LLC on behalf of The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures

References
-
- Hanna M. Novel drugs targeting transthyretin amyloidosis. Curr Heart Fail Rep. 2014;11(1):50‐57. - PubMed
-
- Mohty D, Damy T, Cosnay P, et al. Cardiac amyloidosis: updates in diagnosis and management. Arch Cardiovasc Dis. 2013;106(10):528‐540. - PubMed
-
- Adams D, Gonzalez‐Duarte A, O’Riordan WD, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):11‐21. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

