Recurrent SARS-CoV-2 RNA Detection after COVID-19 Illness Onset during Pregnancy
- PMID: 35213801
- PMCID: PMC8962892
- DOI: 10.3201/eid2804.212354
Recurrent SARS-CoV-2 RNA Detection after COVID-19 Illness Onset during Pregnancy
Abstract
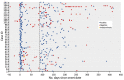
The Surveillance for Emerging Threats to Mothers and Babies Network conducts longitudinal surveillance of pregnant persons in the United States with laboratory-confirmed severe acute respiratory syndrome coronavirus 2 infection during pregnancy. Of 6,551 infected pregnant persons in this analysis, 142 (2.2%) had positive RNA tests >90 days and up to 416 days after infection.
Keywords: 2019 Novel Coronavirus Disease; COVID-19; RT-PCR; SARS-CoV-2; United States; coronavirus disease; pregnancy; real-time reverse transcription PCR; respiratory infections; severe acute respiratory syndrome coronavirus 2; viruses; zoonoses.
Figures
References
-
- Centers for Disease Control and Prevention. Interim guidance on ending isolation and precautions for adults with COVID-19. 2021. Mar 16 [cited 2021 Jun 28]. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

