Rational Development of a Carrier-Free Dry Powder Inhalation Formulation for Respiratory Viral Infections via Quality by Design: A Drug-Drug Cocrystal of Favipiravir and Theophylline
- PMID: 35214034
- PMCID: PMC8876093
- DOI: 10.3390/pharmaceutics14020300
Rational Development of a Carrier-Free Dry Powder Inhalation Formulation for Respiratory Viral Infections via Quality by Design: A Drug-Drug Cocrystal of Favipiravir and Theophylline
Abstract
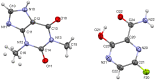
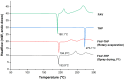
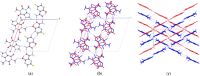
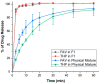
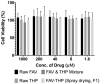
Formulating pharmaceutical cocrystals as inhalable dosage forms represents a unique niche in effective management of respiratory infections. Favipiravir, a broad-spectrum antiviral drug with potential pharmacological activity against SARS-CoV-2, exhibits a low aqueous solubility. An ultra-high oral dose is essential, causing low patient compliance. This study reports a Quality-by-Design (QbD)-guided development of a carrier-free inhalable dry powder formulation containing a 1:1 favipiravir-theophylline (FAV-THP) cocrystal via spray drying, which may provide an alternative treatment strategy for individuals with concomitant influenza infections and chronic obstructive pulmonary disease/asthma. The cocrystal formation was confirmed by single crystal X-ray diffraction, powder X-ray diffraction, and the construction of a temperature-composition phase diagram. A three-factor, two-level, full factorial design was employed to produce the optimized formulation and study the impact of critical processing parameters on the resulting median mass aerodynamic diameter (MMAD), fine particle fraction (FPF), and crystallinity of the spray-dried FAV-THP cocrystal. In general, a lower solute concentration and feed pump rate resulted in a smaller MMAD with a higher FPF. The optimized formulation (F1) demonstrated an MMAD of 2.93 μm and an FPF of 79.3%, suitable for deep lung delivery with no in vitro cytotoxicity observed in A549 cells.
Keywords: SARS-CoV-2; antiviral cocrystal; cocrystal screening; drug-drug cocrystal; improved pharmaceutical properties; inhalable cocrystal; quality-by-design; reformulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Development of favipiravir dry powders for intranasal delivery: An integrated cocrystal and particle engineering approach via spray freeze drying.Int J Pharm. 2024 Mar 25;653:123896. doi: 10.1016/j.ijpharm.2024.123896. Epub 2024 Feb 10. Int J Pharm. 2024. PMID: 38346602
-
Spray-Dried Inhalable Favipiravir Dry Powder Formulation for Influenza Therapy: Preparation and In vivo Evaluation.Curr Drug Deliv. 2025 Mar 12. doi: 10.2174/0115672018351326250306040551. Online ahead of print. Curr Drug Deliv. 2025. PMID: 40077822
-
Optimization, In Vitro, and In Silico Characterization of Theophylline Inhalable Powder Using Raffinose-Amino Acid Combination as Fine Co-Spray-Dried Carriers.Pharmaceutics. 2025 Apr 3;17(4):466. doi: 10.3390/pharmaceutics17040466. Pharmaceutics. 2025. PMID: 40284461 Free PMC article.
-
Synthesis of the first remdesivir cocrystal: design, characterization, and therapeutic potential for pulmonary delivery.Int J Pharm. 2023 Jun 10;640:122983. doi: 10.1016/j.ijpharm.2023.122983. Epub 2023 Apr 29. Int J Pharm. 2023. PMID: 37121494
-
Development of a novel dry powder inhalation formulation for the delivery of rivastigmine hydrogen tartrate.Int J Pharm. 2016 Mar 30;501(1-2):124-38. doi: 10.1016/j.ijpharm.2016.01.066. Epub 2016 Feb 1. Int J Pharm. 2016. PMID: 26836711
Cited by
-
Advanced Drug Delivery Platforms for the Treatment of Oral Pathogens.Pharmaceutics. 2022 Oct 26;14(11):2293. doi: 10.3390/pharmaceutics14112293. Pharmaceutics. 2022. PMID: 36365112 Free PMC article. Review.
-
Pulmonary drug delivery: an effective and convenient delivery route to combat COVID-19.Drug Deliv Transl Res. 2023 Mar;13(3):705-715. doi: 10.1007/s13346-022-01251-1. Epub 2022 Oct 19. Drug Deliv Transl Res. 2023. PMID: 36260223 Free PMC article. Review.
-
Inhaled Dry Powder of Antiviral Agents: A Promising Approach to Treating Respiratory Viral Pathogens.Viruses. 2025 Feb 12;17(2):252. doi: 10.3390/v17020252. Viruses. 2025. PMID: 40007007 Free PMC article. Review.
-
Inhalation Dosage Forms: A Focus on Dry Powder Inhalers and Their Advancements.Pharmaceuticals (Basel). 2023 Nov 28;16(12):1658. doi: 10.3390/ph16121658. Pharmaceuticals (Basel). 2023. PMID: 38139785 Free PMC article. Review.
-
Advancements in Antiviral Therapy: Favipiravir Sodium in Nasal Formulation.AAPS PharmSciTech. 2024 Nov 26;25(8):273. doi: 10.1208/s12249-024-02986-5. AAPS PharmSciTech. 2024. PMID: 39592539
References
-
- World Health Organization WHO Coronavirus Disease (COVID-19) Dashboard. [(accessed on 1 December 2021)]. Available online: https://covid19.who.int/
-
- Centers for Disease Control and Prevention People with Certain Medical Conditions. [(accessed on 1 December 2021)]; Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-....
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

