Poly(Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances
- PMID: 35214091
- PMCID: PMC8877458
- DOI: 10.3390/pharmaceutics14020359
Poly(Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances
Abstract
The sustained release of pharmaceutical substances remains the most convenient way of drug delivery. Hence, a great variety of reports can be traced in the open literature associated with drug delivery systems (DDS). Specifically, the use of microparticle systems has received special attention during the past two decades. Polymeric microparticles (MPs) are acknowledged as very prevalent carriers toward an enhanced bio-distribution and bioavailability of both hydrophilic and lipophilic drug substances. Poly(lactic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA), and their copolymers are among the most frequently used biodegradable polymers for encapsulated drugs. This review describes the current state-of-the-art research in the study of poly(lactic acid)/poly(lactic-co-glycolic acid) microparticles and PLA-copolymers with other aliphatic acids as drug delivery devices for increasing the efficiency of drug delivery, enhancing the release profile, and drug targeting of active pharmaceutical ingredients (API). Potential advances in generics and the constant discovery of therapeutic peptides will hopefully promote the success of microsphere technology.
Keywords: copolymers; drug delivery; drug release mechanisms; microparticles; poly(lactic acid); preparation techniques.
Conflict of interest statement
The authors declare no conflict of interest. The company had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures









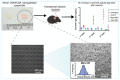






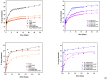

References
-
- Lengyel M., Kállai-Szabó N., Antal V., Laki A.J., Antal I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019;87:20. doi: 10.3390/scipharm87030020. - DOI
-
- Jamaledin R., Sartorius R., Di Natale C., Vecchione R., De Berardinis P., Netti P.A. Recombinant Filamentous Bacteriophages Encapsulated in Biodegradable Polymeric Microparticles for Stimulation of Innate and Adaptive Immune Responses. Microorganisms. 2020;8:650. doi: 10.3390/microorganisms8050650. - DOI - PMC - PubMed
-
- Blasi P. Poly(Lactic Acid)/Poly(Lactic-Co-Glycolic Acid)-Based Microparticles: An Overview. J. Pharm. Investig. 2019;49:337–346. doi: 10.1007/s40005-019-00453-z. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

