The Surface Amine Group of Ultrasmall Magnetic Iron Oxide Nanoparticles Produce Analgesia in the Spinal Cord and Decrease Long-Term Potentiation
- PMID: 35214098
- PMCID: PMC8879719
- DOI: 10.3390/pharmaceutics14020366
The Surface Amine Group of Ultrasmall Magnetic Iron Oxide Nanoparticles Produce Analgesia in the Spinal Cord and Decrease Long-Term Potentiation
Abstract
Our previous studies have revealed the ultrasmall superparamagnetic iron oxide in the amine group USPIO-101 has an analgesic effect on inflammatory pain. Here, we further investigated its effect on the spinal cord and brain via electrophysiological and molecular methods. We used a mouse inflammatory pain model, induced by complete Freund's adjuvant (CFA), and measured pain thresholds via von Frey methods. We also investigated the effects of USPIO-101 via an extracellular electrophysiological recording at the spinal dorsal horn synapses and hippocampal Schaffer collateral-CA1 synapses, respectively. The mRNA expression of pro-inflammatory cytokines was detected by quantitative real-time polymerase chain reaction (RT-qPCR). Our results showed intrathecal USPIO-101 produces similar analgesic behavior in mice with chronic inflammatory pain via intrathecal or intraplantar administration. The potentiated low-frequency stimulation-induced spinal cord long-term potentiation (LTP) at the spinal cord superficial dorsal horn synapses could decrease via USPIO-101 in mice with chronic inflammatory pain. However, the mRNA expression of cyclooxygenase-2 was enhanced with lipopolysaccharide (LPS) stimulation in microglial cells, and we also found USPIO-101 at 30 µg/mL could decrease the magnitude of hippocampal LTP. These findings revealed that intrathecal USPIO-101 presented an analgesia effect at the spinal cord level, but had neurotoxicity risk at higher doses.
Keywords: analgesia; inflammatory pain; long-term potentiation; neurotoxicity; pro-inflammatory cytokines; ultrasmall magnetic iron oxide nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




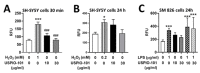


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

