Pharmaceutical and Safety Profile Evaluation of Novel Selenocompounds with Noteworthy Anticancer Activity
- PMID: 35214099
- PMCID: PMC8875489
- DOI: 10.3390/pharmaceutics14020367
Pharmaceutical and Safety Profile Evaluation of Novel Selenocompounds with Noteworthy Anticancer Activity
Abstract
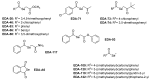
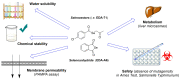
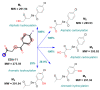
Prior studies have reported the potent and selective cytotoxic, pro-apoptotic, and chemopreventive activities of a cyclic selenoanhydride and of a series of selenoesters. Some of these selenium derivatives demonstrated multidrug resistance (MDR)-reversing activity in different resistant cancer cell lines. Thus, the aim of this study was to evaluate the pharmaceutical and safety profiles of these selected selenocompounds using alternative methods in silico and in vitro. One of the main tasks of this work was to determine both the physicochemical properties and metabolic stability of these selenoesters. The obtained results proved that these tested selenocompounds could become potential candidates for novel and safe anticancer drugs with good ADMET parameters. The most favorable selenocompounds turned out to be the phthalic selenoanhydride (EDA-A6), two ketone-containing selenoesters with a 4-chlorophenyl moiety (EDA-71 and EDA-73), and a symmetrical selenodiester with a pyridine ring and two selenium atoms (EDA-119).
Keywords: ADMET; Ames test; PAMPA; anticancer activity; metabolic stability; pharmaceutical profile; selenoesters.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

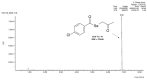
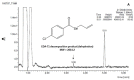

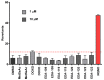
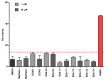
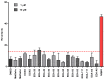
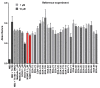
References
Grants and funding
LinkOut - more resources
Full Text Sources

