Sodium Valproate Incompatibility with Parenteral Nutrition Admixtures-A Risk to Patient Safety: An In Vitro Evaluation Study
- PMID: 35214103
- PMCID: PMC8876349
- DOI: 10.3390/pharmaceutics14020371
Sodium Valproate Incompatibility with Parenteral Nutrition Admixtures-A Risk to Patient Safety: An In Vitro Evaluation Study
Abstract
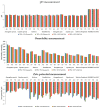
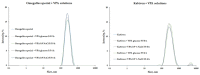
Epilepsy is defined as a group of concerning problems related to the nervous system; its defining feature is a predisposition to epileptic seizures. The frequency of seizures in intensive care units (ICU) ranges from 3.3% to 34%, and ICU antiepileptic treatment is routine practice. The administration of drugs through the same infusion line is not recommended but is common clinical practice, especially in ICU. Incompatibilities between parenteral drugs and between drugs and parenteral nutrition admixtures (PNAs) are common medical errors and pose risks to patient safety. The co-administration of drugs must always be confirmed and clearly defined. The simultaneous infusion of sodium valproate (VPA, drug used to treat seizures and epilepsy) with parenteral PNAs has not yet been studied. During the experiment reported in this study, a visual control, pH, osmolality, zeta potential, particle size, polydispersity index, and turbidity were measured. The conducted research shows that the lipid emulsion composition has a significant influence on drug-PN (drug-parenteral nutrition) compatibility. The acceptance criteria were met only for PNs containing omega-3-acid-triglycerides (Omegaflex special and peri). The second fraction of particles above 1000 nm was observed for most of the tested PNAs (Lipoflex special, Lipoflex peri, Kabiven, SmofKabiven, Kabiven Peripheral, and Olimel Peri N4E), which disqualifies their simultaneous administration with VPA.
Keywords: compatibility; interaction; medical errors; safe therapy; sodium valproate.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Potential Use of Common Administration of Emulsion for Parenteral Nutrition and Vinpocetine: Compatibility Study and Prospect.Metabolites. 2024 Aug 7;14(8):439. doi: 10.3390/metabo14080439. Metabolites. 2024. PMID: 39195535 Free PMC article.
-
Y-Site Compatibility Studies of Ketoprofen with Parenteral Nutrition Admixtures for Central and Peripheral Administration.Pharmaceutics. 2022 Nov 23;14(12):2570. doi: 10.3390/pharmaceutics14122570. Pharmaceutics. 2022. PMID: 36559064 Free PMC article.
-
In vitro compatibility studies of vancomycin with ready-to-use parenteral nutrition admixtures for safer clinical practice.Clin Nutr. 2020 Aug;39(8):2539-2546. doi: 10.1016/j.clnu.2019.11.014. Epub 2019 Nov 15. Clin Nutr. 2020. PMID: 31784302
-
Y-Site Compatibility Studies of Parenteral Nutrition and Other Intravenous Medications in Neonatal and Pediatric Patients: A Review of the Literature Evidence.Pharmaceutics. 2024 Feb 12;16(2):264. doi: 10.3390/pharmaceutics16020264. Pharmaceutics. 2024. PMID: 38399318 Free PMC article. Review.
-
Clinical nutrition and drug interactions.Ulus Cerrahi Derg. 2013 Nov 14;29(4):177-86. doi: 10.5152/UCD.2013.112013. eCollection 2013. Ulus Cerrahi Derg. 2013. PMID: 25931873 Free PMC article. Review.
Cited by
-
Potential Use of Common Administration of Emulsion for Parenteral Nutrition and Vinpocetine: Compatibility Study and Prospect.Metabolites. 2024 Aug 7;14(8):439. doi: 10.3390/metabo14080439. Metabolites. 2024. PMID: 39195535 Free PMC article.
-
Y-Site Compatibility Studies of Ketoprofen with Parenteral Nutrition Admixtures for Central and Peripheral Administration.Pharmaceutics. 2022 Nov 23;14(12):2570. doi: 10.3390/pharmaceutics14122570. Pharmaceutics. 2022. PMID: 36559064 Free PMC article.
-
Physicochemical Compatibility of Ceftolozane-Tazobactam with Parenteral Nutrition.Pharmaceuticals (Basel). 2024 Jul 5;17(7):896. doi: 10.3390/ph17070896. Pharmaceuticals (Basel). 2024. PMID: 39065746 Free PMC article.
-
Antiemetic Drugs Compatibility Evaluation with Paediatric Parenteral Nutrition Admixtures.Pharmaceutics. 2023 Aug 15;15(8):2143. doi: 10.3390/pharmaceutics15082143. Pharmaceutics. 2023. PMID: 37631357 Free PMC article.
-
The Modern Approach to Total Parenteral Nutrition: Multidirectional Therapy Perspectives with a Focus on the Physicochemical Stability of the Lipid Fraction.Nutrients. 2025 Feb 28;17(5):846. doi: 10.3390/nu17050846. Nutrients. 2025. PMID: 40077716 Free PMC article. Review.
References
-
- Berg A.T., Berkovic S.F., Brodie M.J., Buchhalter J., Cross J.H., van Emde Boas W., Engel J., French J., Glauser T.A., Mathern G.W., et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. - DOI - PubMed
-
- World Health Organization Epilepsy. [(accessed on 20 December 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy.
-
- Healthcare Cost and Utilization Project. [(accessed on 20 December 2021)]; Available online: https://hcupnet.ahrq.gov/#setup.
LinkOut - more resources
Full Text Sources
Miscellaneous

