Potent Antiplasmodial Derivatives of Dextromethorphan Reveal the Ent-Morphinan Pharmacophore of Tazopsine-Type Alkaloids
- PMID: 35214104
- PMCID: PMC8876632
- DOI: 10.3390/pharmaceutics14020372
Potent Antiplasmodial Derivatives of Dextromethorphan Reveal the Ent-Morphinan Pharmacophore of Tazopsine-Type Alkaloids
Abstract
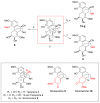
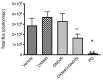
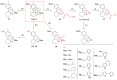
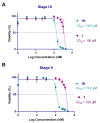
The alkaloid tazopsine 1 was introduced in the late 2000s as a novel antiplasmodial hit compound active against Plasmodium falciparum hepatic stages, with the potential to develop prophylactic drugs based on this novel chemical scaffold. However, the structural determinants of tazopsine 1 bioactivity, together with the exact definition of the pharmacophore, remained elusive, impeding further development. We found that the antitussive drug dextromethorphan (DXM) 3, although lacking the complex pattern of stereospecific functionalization of the natural hit, was harboring significant antiplasmodial activity in vitro despite suboptimal prophylactic activity in a murine model of malaria, precluding its direct repurposing against the disease. The targeted N-alkylation of nor-DXM 15 produced a small library of analogues with greatly improved activity over DXM 3 against P. falciparum asexual stages. Amongst these, N-2'-pyrrolylmethyl-nor-DXM 16i showed a 2- to 36-fold superior inhibitory potency compared to tazopsine 1 and DXM 3 against P. falciparum liver and blood stages, with respectively 760 ± 130 nM and 2.1 ± 0.4 μM IC50 values, as well as liver/blood phase selectivity of 2.8. Furthermore, cpd. 16i showed a 5- to 8-fold increase in activity relative to DXM 3 against P. falciparum stages I-II and V gametocytes, with 18.5 μM and 13.2 μM IC50 values, respectively. Cpd. 16i can thus be considered a promising novel hit compound against malaria in the ent-morphinan series with putative pan cycle activity, paving the way for further therapeutic development (e.g., investigation of its prophylactic activity in vivo).
Keywords: N-alkylation; Plasmodium berghei; Plasmodium falciparum; blood stages; dextromethorphan; hepatic stages; hit compound; malaria; prophylaxis; tazopsine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- WHO . World Malaria Report. WHO; Geneva, Switzerland: 2019.
-
- Ritchie H., Roser M., Causes of Death Our World in Data 2018. [(accessed on 28 December 2021)]. Available online: https://ourworldindata.org/causes-of-death.
-
- Imwong M., Suwannasin K., Kunasol C., Sutawong K., Mayxay M., Rekol H., Smithuis F.M., Hlaing T.M., Tun K.M., van der Pluijm R.W., et al. The spread of artemisinin-resistant Plasmodium falciparum in the Greater Mekong subregion: A molecular epidemiology observational study. Lancet Infect. Dis. 2017;17:491–497. doi: 10.1016/S1473-3099(17)30048-8. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

