Linkers: An Assurance for Controlled Delivery of Antibody-Drug Conjugate
- PMID: 35214128
- PMCID: PMC8874516
- DOI: 10.3390/pharmaceutics14020396
Linkers: An Assurance for Controlled Delivery of Antibody-Drug Conjugate
Abstract
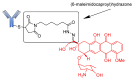
As one of the major therapeutic options for cancer treatment, chemotherapy has limited selectivity against cancer cells. Consequently, this therapeutic strategy offers a small therapeutic window with potentially high toxicity and thus limited efficacy of doses that can be tolerated by patients. Antibody-drug conjugates (ADCs) are an emerging class of anti-cancer therapeutic drugs that can deliver highly cytotoxic molecules directly to cancer cells. To date, twelve ADCs have received market approval, with several others in clinical stages. ADCs have become a powerful class of therapeutic agents in oncology and hematology. ADCs consist of recombinant monoclonal antibodies that are covalently bound to cytotoxic chemicals via synthetic linkers. The linker has a key role in ADC outcomes because its characteristics substantially impact the therapeutic index efficacy and pharmacokinetics of these drugs. Stable linkers and ADCs can maintain antibody concentration in blood circulation, and they do not release the cytotoxic drug before it reaches its target, thus resulting in minimum off-target effects. The linkers used in ADC development can be classified as cleavable and non-cleavable. The former, in turn, can be grouped into three types: hydrazone, disulfide, or peptide linkers. In this review, we highlight the various linkers used in ADC development and their design strategy, release mechanisms, and future perspectives.
Keywords: FDA; antibody-drug conjugates (ADCs); bioconjugation; chemotherapy; cytotoxic drug; linker; monoclonal antibody; tumor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






















References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

