Anticancer Activity of the Choline Kinase Inhibitor PL48 Is Due to Selective Disruption of Choline Metabolism and Transport Systems in Cancer Cell Lines
- PMID: 35214160
- PMCID: PMC8876215
- DOI: 10.3390/pharmaceutics14020426
Anticancer Activity of the Choline Kinase Inhibitor PL48 Is Due to Selective Disruption of Choline Metabolism and Transport Systems in Cancer Cell Lines
Abstract
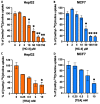
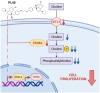
A large number of different types of cancer have been shown to be associated with an abnormal metabolism of phosphatidylcholine (PC), the main component of eukaryotic cell membranes. Indeed, the overexpression of choline kinase α1 (ChoKα1), the enzyme that catalyses the bioconversion of choline to phosphocholine (PCho), has been found to associate with cell proliferation, oncogenic transformation and carcinogenesis. Hence, ChoKα1 has been described as a possible cancer therapeutic target. Moreover, the choline transporter CTL1 has been shown to be highly expressed in several tumour cell lines. In the present work, we evaluate the antiproliferative effect of PL48, a rationally designed inhibitor of ChoKα1, in MCF7 and HepG2 cell lines. In addition, we illustrate that the predominant mechanism of cellular choline uptake in these cells is mediated by the CTL1 choline transporter. A possible correlation between the inhibition of both choline uptake and ChoKα1 activity and cell proliferation in cancer cell lines is also highlighted. We conclude that the efficacy of this inhibitor on cell proliferation in both cell lines is closely correlated with its capability to block choline uptake and ChoKα1 activity, making both proteins potential targets in cancer therapy.
Keywords: cancer; choline kinase inhibitors; choline uptake; lipid metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
LinkOut - more resources
Full Text Sources
Research Materials

