P-Type Metal Oxide Semiconductor Thin Films: Synthesis and Chemical Sensor Applications
- PMID: 35214257
- PMCID: PMC8963036
- DOI: 10.3390/s22041359
P-Type Metal Oxide Semiconductor Thin Films: Synthesis and Chemical Sensor Applications
Abstract
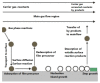
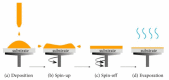
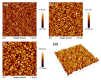
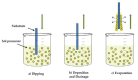
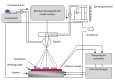
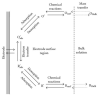
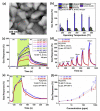
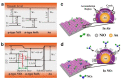
This review focuses on the synthesis of p-type metal-oxide (p-type MOX) semiconductor thin films, such as CuO, NiO, Co3O4, and Cr2O3, used for chemical-sensing applications. P-type MOX thin films exhibit several advantages over n-type MOX, including a higher catalytic effect, low humidity dependence, and improved recovery speed. However, the sensing performance of CuO, NiO, Co3O4, and Cr2O3 thin films is strongly related to the intrinsic physicochemical properties of the material and the thickness of these MOX thin films. The latter is heavily dependent on synthesis techniques. Many techniques used for growing p-MOX thin films are reviewed herein. Physical vapor-deposition techniques (PVD), such as magnetron sputtering, thermal evaporation, thermal oxidation, and molecular-beam epitaxial (MBE) growth were investigated, along with chemical vapor deposition (CVD). Liquid-phase routes, including sol-gel-assisted dip-and-spin coating, spray pyrolysis, and electrodeposition, are also discussed. A review of each technique, as well as factors that affect the physicochemical properties of p-type MOX thin films, such as morphology, crystallinity, defects, and grain size, is presented. The sensing mechanism describing the surface reaction of gases with MOX is also discussed. The sensing characteristics of CuO, NiO, Co3O4, and Cr2O3 thin films, including their response, sensor kinetics, stability, selectivity, and repeatability are reviewed. Different chemical compounds, including reducing gases (such as volatile organic compounds (VOCs), H2, and NH3) and oxidizing gases, such as CO2, NO2, and O3, were analyzed. Bulk doping, surface decoration, and heterostructures are some of the strategies for improving the sensing capabilities of the suggested pristine p-type MOX thin films. Future trends to overcome the challenges of p-type MOX thin-film chemical sensors are also presented.
Keywords: CVD; PVD; chemical sensors; liquid-phase route; p-type metal-oxide semiconductors; synthesis techniques; thin films.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


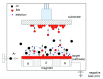
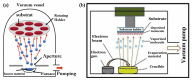
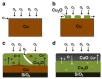
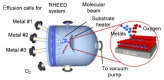







References
-
- Kishore Kumar D., Raghava Reddy K., Sadhu V., Shetti N.P., Venkata Reddy C., Chouhan R.S., Naveen S. Metal Oxide-Based Nanosensors for Healthcare and Environmental Applications. Elsevier; Amsterdam, The Netherlands: 2020.
-
- Shi Y., Xu H., Liu T., Zeb S., Nie Y., Zhao Y., Qin C., Jiang X. Advanced development of metal oxide nanomaterials for H2 gas sensing applications. Mater. Adv. 2021;2:1530–1569. doi: 10.1039/D0MA00880J. - DOI
-
- Moumen A., Zappa D., Poli N., Comini E. Catalyst–Assisted vapor liquid solid growth of α-Bi2O3 nanowires for acetone and ethanol detection. Sens. Actuators B Chem. 2021;346:130432. doi: 10.1016/j.snb.2021.130432. - DOI
-
- Barsan N., Koziej D., Weimar U. Metal oxide-based gas sensor research: How to? Sens. Actuators B Chem. 2007;121:18–35. doi: 10.1016/j.snb.2006.09.047. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

