The Population-Wide Risk-Benefit Profile of Extending the Primary COVID-19 Vaccine Course Compared with an mRNA Booster Dose Program
- PMID: 35214599
- PMCID: PMC8880242
- DOI: 10.3390/vaccines10020140
The Population-Wide Risk-Benefit Profile of Extending the Primary COVID-19 Vaccine Course Compared with an mRNA Booster Dose Program
Abstract
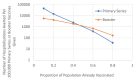
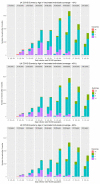
The vaccination program is reducing the burden of COVID-19. However, recently, COVID-19 infections have been increasing across Europe, providing evidence that vaccine efficacy is waning. Consequently, booster doses are required to restore immunity levels. However, the relative risk-benefit ratio of boosters, compared to pursuing a primary course in the unvaccinated population, remains uncertain. In this study, a susceptible-exposed-infectious-recovered (SEIR) transmission model of SARS-CoV-2 was used to investigate the impact of COVID-19 vaccine waning on disease burden, the benefit of a booster vaccine program compared to targeting the unvaccinated population, and the population-wide risk-benefit profile of vaccination. Our data demonstrates that the rate of vaccine efficacy waning has a significant impact on COVID-19 hospitalisations with the greatest effect in populations with lower vaccination coverage. There was greater benefit associated with a booster vaccination strategy compared to targeting the unvaccinated population, once >50% of the population had received their primary vaccination course. The population benefits of vaccination (reduced hospitalisations, long-COVID and deaths) outweighed the risks of myocarditis/pericarditis by an order of magnitude. Vaccination is important in ending the COVID-19 pandemic sooner, and the reduction in hospitalisations, death and long-COVID associated with vaccination significantly outweigh any risks. Despite these obvious benefits some people are vaccine reluctant, and as such remain unvaccinated. However, when most of a population have been vaccinated, a focus on a booster vaccine strategy for this group is likely to offer greater value, than targeting the proportion of the population who choose to remain unvaccinated.
Keywords: COVID-19; boosters; modelling; population risk.
Conflict of interest statement
T.S., A.R.M. and P.M. are employees of Health Economics and Outcomes Research Ltd., Cardiff, UK. Health Economics and Outcomes Research Ltd. received fees from Moderna in relation to this study. C.A.T. and P.O.B. are employees of Moderna, Inc. and hold Moderna stocks/stock options. M.M. is an employee of Ashfield Healthcare on behalf of Moderna and does not hold stocks/ stock options. M.E. and W.D.S. have no conflicts of interest to declare.
Figures


References
-
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. - DOI - PMC - PubMed
-
- Shrotri M., Krutikov M., Palmer T., Giddings R., Azmi B., Subbarao S., Fuller C., Irwin-Singer A., Davies D., Tut G., et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): A prospective cohort study. Lancet Infect. Dis. 2021;21:1529–1538. doi: 10.1016/S1473-3099(21)00289-9. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

