Effectiveness of Vaccination against SARS-CoV-2 Infection in the Pre-Delta Era: A Systematic Review and Meta-Analysis
- PMID: 35214615
- PMCID: PMC8879968
- DOI: 10.3390/vaccines10020157
Effectiveness of Vaccination against SARS-CoV-2 Infection in the Pre-Delta Era: A Systematic Review and Meta-Analysis
Abstract
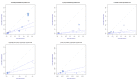
(1) Background: The objective of this study was to assess the effectiveness of SARS-CoV-2 vaccines in terms of prevention of disease and transmission in the pre-Delta era. The evaluation was narrowed to two mRNA vaccines and two modified adenovirus-vectored vaccines. (2) Methods: The overall risk of any SARS-CoV-2 infection confirmed by positive real-time Polymerase Chain Reaction (PCR) test was estimated in partially and fully vaccinated individuals. The evidence synthesis was pursued through a random-effects meta-analysis. The effect size was expressed as relative risk (RR) and RRR (RR reduction) of SARS-CoV-2 infection following vaccination. Heterogeneity was investigated through a between-study heterogeneity analysis and a subgroup meta-analysis. (3) Results: The systematic review identified 27 studies eligible for the quantitative synthesis. Partially vaccinated individuals presented a RRR = 73% (95%CI = 59-83%) for positive SARS-CoV-2 PCR (RR = 0.27) and a RRR=79% (95%CI = 30-93%) for symptomatic SARS-CoV-2 PCR (RR = 0.21). Fully vaccinated individuals showed a RRR = 94% (95%CI = 88-98%) for SARS-CoV-2 positive PCR (RR = 0.06) compared to unvaccinated individuals. The full BNT162b2 vaccination protocol achieved a RRR = 84-94% against any SARS-CoV-2-positive PCR and a RRR = 68-84% against symptomatic positive PCR. (4) Conclusions: The meta-analysis results suggest that full vaccination might block transmission. In particular, the risk of SARS-CoV-2 infection appeared higher for non-B.1.1.7 variants and individuals aged ≥69 years. Considering the high level of heterogeneity, these findings must be taken with caution. Further research on SARS-CoV-2 vaccine effectiveness against emerging SARS-CoV-2 variants is encouraged.
Keywords: SARS-CoV-2 infection; coronavirus disease 2019; vaccine effectiveness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
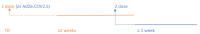



References
-
- COVID19 Database. [(accessed on 13 July 2021)]. Available online: https://COVID19.who.int/
-
- Peng Y., Mentzer A.J., Liu G., Yao X., Yin Z., Dong D., Dejnirattisai W., Rostron T., Supasa P., Liu C., et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 2020;21:1336–1345. doi: 10.1038/s41590-020-0782-6. - DOI - PMC - PubMed
-
- Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., Voysey M., Aley P.K., Angus B., Babbage G., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. - DOI - PMC - PubMed
-
- EMA. [(accessed on 13 July 2021)]. Available online: https://www.ema.europa.eu/en/medicines.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

