Antibody Response of Combination of BNT162b2 and CoronaVac Platforms of COVID-19 Vaccines against Omicron Variant
- PMID: 35214619
- PMCID: PMC8877145
- DOI: 10.3390/vaccines10020160
Antibody Response of Combination of BNT162b2 and CoronaVac Platforms of COVID-19 Vaccines against Omicron Variant
Abstract
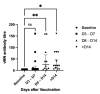
By vaccinating SARS-CoV-2 naïve individuals who have already received two doses of COVID-19 vaccines, we aimed to investigate whether a heterologous prime-boost strategy, using vaccines of different platforms as the booster dose, can enhance the immune response against SARS-CoV-2 virus variants. Participants were assigned into four groups, each receiving different combination of vaccinations: two doses of BNT162b2 followed by one dose of BNT162b2 booster (B-B-B); Combination of BNT162b2 (first dose) and CoronaVac (second dose) followed by one dose of BNT162b2 booster (B-C-B); two doses of CoronaVac followed by one dose of CoronaVac booster (C-C-C); two doses of CoronaVac followed by one dose of BNT162b2 booster (C-C-B). The neutralizing antibody in sera against the virus was determined with live virus microneutralization assay (vMN). The B-B-B group and C-C-B group demonstrated significantly higher immunogenicity against SARS-CoV-2 Wild type (WT), Beta variant (BV) and Delta variant (DV). In addition, the B-B-B group and C-C-B group showed reduced but existing protection against Omicron variant (OV). Moreover, A persistent rise in vMN titre against OV was observed 3 days after booster dose. Regarding safety, a heterologous prime-boost vaccine strategy is well tolerated. In this study, it was demonstrated that using vaccines of different platforms as booster dose can enhance protection against SARS-CoV-2 variants, offering potent neutralizing activity against wild-type virus (WT), Beta variant (BV), Delta variant (DV) and some protection against the Omicron variant (OV). In addition, a booster mRNA vaccine results in a more potent immune response than inactivated vaccine regardless of which platform was used for prime doses.
Keywords: COVID-19; omicron variant; vaccines.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., Han W., Chen Z., Tang R., Yin W., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. - DOI - PMC - PubMed
-
- Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., Bellamy D., Bibi S., Bittaye M., Clutterbuck E.A., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. - DOI - PMC - PubMed
-
- Richmond P., Hatchuel L., Dong M., Ma B., Hu B., Smolenov I., Li P., Liang P., Han H.H., Liang J., et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: A phase 1, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397:682–694. doi: 10.1016/S0140-6736(21)00241-5. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

