Determinants of the Development of SARS-CoV-2 Anti-Spike Immune-Response after Vaccination among Healthcare Workers in Egypt
- PMID: 35214633
- PMCID: PMC8878288
- DOI: 10.3390/vaccines10020174
Determinants of the Development of SARS-CoV-2 Anti-Spike Immune-Response after Vaccination among Healthcare Workers in Egypt
Abstract
Background: Understanding the factors affecting humoral immune response to COVID-19 vaccines among healthcare workers (HCWs) is essential to predict their level of protection. Vaccination elicits antibodies against SARS-CoV-2 spike protein (anti-S).
Aim: To investigate the factors associated with the presence of SARS-CoV-2 anti-S antibodies among vaccinated HCWs.
Methods: This cross-sectional study included 143 vaccinated HCWs, with or without a history of previous COVID-19 infection (clinically, radiologically, or by laboratory results) from different departments. Socio-demographic, clinical, as well as vaccine-related data, were recorded. Serum samples were collected and tested for SARS-CoV-2 spike antibodies.
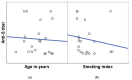
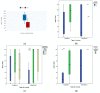
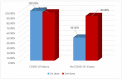
Results: Vaccination provoked an immunogenic response, where the overall anti-S positivity was 83.9% (95% CI: 77.8-90.0%). The response was not affected either by the age or gender of HCWs. Out of the 143 HCWs, 46 (32.1%; 95% CI: 24.4-39.9%) reported a previous history of COVID-19 infection, and seropositivity was significantly higher among them (p = 0.002), and it was associated with the frequency of infection (p = 0.044) and duration since diagnosis of COVID-19 infection (p = 0.065). They had higher median anti-S titers (111.8 RU/mL) than those without infection (39.8 RU/mL). Higher seropositivity was observed with Oxford/AstraZeneca vaccine (AZD1222) (88.9%; 95% CI: 83.1-95.0%) than Sinopharm (BBIBP-CorV) (67.7%; 95% CI: 50.3-85.2%), and with receiving two doses of vaccine (92.3%; 95% CI: 87.1-97.5%).
Conclusions: Antibody positivity was significantly affected by the previous history of COVID-19 infection, type of vaccine, the number of doses received, and duration since vaccination.
Keywords: SARS-CoV-2 anti-spike antibody; healthcare workers; immune response; post-vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- World Health Organization Prevention, Identification and Management of Health Worker Infection in the Context of COVID-19. [(accessed on 15 December 2021)]. Available online: https://www.who.int/publications/i/item/10665-336265.
-
- World Health Organization. [(accessed on 10 December 2021)]. Available online: https://apps.who.int/iris/handle/10665/345300.
-
- Health Workers in Focus: Policies and Practices for Successful Public Response to COVID-19 Vaccination. Strategic Considerations for the Member States in the WHO European Region. WHO Regional Office for Europe; Copenhagen, Denmark: 2021. License: CC BY-NC-SA 3.0 IGO.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

