Cost-Effectiveness and Burden of Disease for Adjuvanted Quadrivalent Influenza Vaccines Compared to High-Dose Quadrivalent Influenza Vaccines in Elderly Patients in Spain
- PMID: 35214635
- PMCID: PMC8879805
- DOI: 10.3390/vaccines10020176
Cost-Effectiveness and Burden of Disease for Adjuvanted Quadrivalent Influenza Vaccines Compared to High-Dose Quadrivalent Influenza Vaccines in Elderly Patients in Spain
Abstract
Influenza is a contagious respiratory disease that causes severe illness and death, particularly in elderly populations. Two enhanced formulations of quadrivalent influenza vaccine (QIV) are available in Spain. Adjuvanted QIV (aQIV) is available for those aged 65+ and high-dose QIV (HD-QIV) for those aged 60+. In this study, we used a health economic model to assess the costs and outcomes associated with using aQIV or HD-QIV in subjects aged 65+. Using aQIV instead of HD-QIV to vaccinate an estimated 5,126,343 elderly people results in reductions of 5405 symptomatic cases, 760 primary care visits, 171 emergency room visits, 442 hospitalizations, and 26 deaths in Spain each year. Life-years (LYs) and quality-adjusted LYs (QALYs) increases by 260 and 206, respectively, each year. Savings from a direct medical payer perspective are EUR 63.6 million, driven by the lower aQIV vaccine price and a minor advantage in effectiveness. From a societal perspective, savings increase to EUR 64.2 million. Results are supported by scenario and sensitivity analyses. When vaccine prices are assumed equal, aQIV remains dominant compared to HD-QIV. Potential savings are estimated at over EUR 61 million in vaccine costs alone. Therefore, aQIV provides a highly cost-effective alternative to HD-QIV for people aged 65+ in Spain.
Keywords: Spain; adjuvanted; burden of illness; cost-effectiveness; high dose; influenza; vaccination.
Conflict of interest statement
Ray Gani, Richard Guerrero-Luduena, and Piedad Alvarez are salaried employees of Evidera and are not allowed to accept remuneration from any clients for their services. Jesús Ruiz-Aragón and Sergio Márquez received consultancy fees from Evidera to conduct the study and develop this manuscript.
Figures

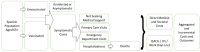

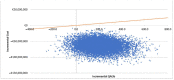
References
-
- World Health Organization Influenza (Seasonal) 2018. [(accessed on 1 November 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
-
- Nielsen J., Vestergaard L.S., Richter L., Schmid D., Bustos N., Asikainen T., Trebbien R., Denissov G., Innos K., Virtanen M.J., et al. European all-cause excess and influenza-attributable mortality in the 2017/18 season: Should the burden of influenza B be reconsidered? Clin. Microbiol. Infect. 2019;25:1266–1276. doi: 10.1016/j.cmi.2019.02.011. - DOI - PubMed
-
- Ministerio de Sanidad Consumo y Bienestar Social Recomendaciones de Vacunación Frente a la Gripe. 2021–2022. [(accessed on 1 November 2021)]. Available online: https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacuna....
-
- World Health Organization Recommended Composition of Influenza Virus Vaccines for Use in the 2022 Southern Hemisphere Influenza Season. 2021. [(accessed on 1 November 2021)]. Available online: https://cdn.who.int/media/docs/default-source/influenza/who-influenza-re....
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

