Low Neutralizing Antibody Titers against the Mu Variant of SARS-CoV-2 in 31 BNT162b2 Vaccinated Individuals in Colombia
- PMID: 35214639
- PMCID: PMC8876570
- DOI: 10.3390/vaccines10020180
Low Neutralizing Antibody Titers against the Mu Variant of SARS-CoV-2 in 31 BNT162b2 Vaccinated Individuals in Colombia
Abstract
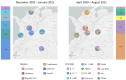
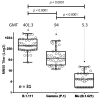
Global surveillance programs for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are showing the emergence of variants with mutations in the spike protein. Genomic and laboratory surveillance are important to determine if these variants may be more infectious or less susceptible to antiviral treatments and vaccine-induced antibodies. Three of the most predominant SARS-CoV-2 variants in Colombia during the epidemiological peaks of 2021 were isolated: Mu, a variant of interest; Gamma, a variant of concern; B.1.111, which lacks genetic markers associated with greater virulence. Microneutralization assays were performed by incubating 120 mean tissue culture infectious doses (TCID50) of each SARS-CoV-2 isolate with five two-fold serial dilutions of sera from 31 BNT162b2-vaccinated volunteers. The mean neutralization titer (MN50) was calculated by the Reed-Muench method. At the end of August, Mu represented 49% of coronavirus disease 2019 (COVID-19) cases in Colombia, followed by 25% of Gamma. In contrast, B.1.111 became almost undetectable. The evaluation of neutralizing antibodies suggests that patients vaccinated with BNT162b2 generate neutralizing antibody titers against the Mu variant at significantly lower concentrations relative to B.1.111 and Gamma. This study shows the importance of continuing surveillance programs of emerging variants, as well as the need to evaluate the neutralizing antibody response induced by other vaccines.
Keywords: COVID-19; Mu (B.1.621) variant; SARS-CoV-2 variants; gamma (P.1) variant; neutralizing antibodies; spike protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Rotondo J.C., Martini F., Maritati M., Mazziotta C., Di Mauro G., Lanzillotti C., Barp N., Gallerani A., Tognon M., Contini C. SARS-CoV-2 infection: New molecular, phylogenetic, and pathogenetic insights. Efficacy of current vaccines and the potential risk of variants. Viruses. 2021;13:1687. doi: 10.3390/v13091687. - DOI - PMC - PubMed
-
- Aleem A., Akbar Samad A.B., Slenker A.K. Emerging Variants of SARS-CoV-2 and Novel Therapeutics against Coronavirus (COVID-19) StatPearls Publishing; Treasure Island, FL, USA: 2021. - PubMed
-
- Álvarez-Díaz D.A., Laiton-Donato K., Torres-García O.A., Ruiz-Moreno H.A., Franco-Muñoz C., Beltran M.A., Mercado-Reyes M., Rueda M.G., Muñoz A.L. Reduced levels of convalescent neutralizing antibodies against SARS-CoV-2 B.1+L249S+E484K lineage. Virus Res. 2022;308:198629. doi: 10.1016/j.virusres.2021.198629. - DOI - PMC - PubMed
-
- WHO Tracking SARS-CoV-2 Variants. [(accessed on 27 September 2021)]. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

