Oncolytic Vaccinia Virus in Lung Cancer Vaccines
- PMID: 35214699
- PMCID: PMC8875327
- DOI: 10.3390/vaccines10020240
Oncolytic Vaccinia Virus in Lung Cancer Vaccines
Abstract
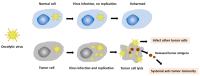
Therapeutic cancer vaccines represent a promising therapeutic modality via the induction of long-term immune response and reduction in adverse effects by specifically targeting tumor-associated antigens. Oncolytic virus, especially vaccinia virus (VV) is a promising cancer treatment option for effective cancer immunotherapy and thus can also be utilized in cancer vaccines. Non-small cell lung cancer (NSCLC) is likely to respond to immunotherapy, such as immune checkpoint inhibitors or cancer vaccines, since it has a high tumor mutational burden. In this review, we will summarize recent applications of VV in lung cancer treatment and discuss the potential and direction of VV-based therapeutic vaccines.
Keywords: cancer vaccine; immunotherapy; non-small cell lung cancer; oncolytic virus; personalized vaccination; vaccinia virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Cáceres-Lavernia H.H., Nenínger-Vinageras E., Varona-Rodríguez L.M., Olivares-Romero Y.A., Sánchez-Rojas I., Mazorra-Herrera Z., Basanta-Bergolla D., Duvergel-Calderín D., Torres-Cuevas B.L., del Castillo-Carrillo C. Racotumomab in Non-Small Cell Lung Cancer as Maintenance and Second-Line Treatment. MEDICC Rev. 2021;23:21–28. doi: 10.37757/MR2021.V23.N3.5. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

