Safety, Immunogenicity and Lot-to-Lot Consistency of Sabin-Strain Inactivated Poliovirus Vaccine in 2-Month-Old Infants: A Double-Blind, Randomized Phase III Trial
- PMID: 35214712
- PMCID: PMC8879689
- DOI: 10.3390/vaccines10020254
Safety, Immunogenicity and Lot-to-Lot Consistency of Sabin-Strain Inactivated Poliovirus Vaccine in 2-Month-Old Infants: A Double-Blind, Randomized Phase III Trial
Abstract
Background: The Sabin-strain-based inactivated poliovirus vaccine (sIPV) plays an important role in poliomyelitis eradication in developing countries. As part of the phase III clinical development program, this study aimed to evaluate the safety, immunogenicity and lot-to-lot consistency of the sIPV in 2-month-old infants.
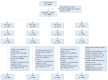
Method: We conducted a phase III, randomized, double-blind, positive-controlled trial in which 1300 healthy infants were randomly assigned to four groups in a 1:1:1:1 ratio to receive one of the three lots of the sIPV or the control IPV at 2, 3 and 4 months of age. Serum samples were collected before the first dose and 30 days after the third dose of vaccination to assess the immunogenicity. Solicited local and systemic reactions were recorded within 7 days and unsolicited adverse events within 30 days after each vaccination.
Results: Of the 1300 randomized infants, 1190 infants completed the study and were included in the per-protocol population. The seroconversion rates in the three lots of the sIPV were 95.67%, 97.03% and 95.59%, respectively, for type 1; 94.33%, 93.73% and 92.88%, respectively, for type 2; and 98.67%, 99.67% and 99.32%, respectively, for type 3. The ratios of GMTs for poliovirus types 1, 2 and 3 of each pair of lots were all between 0.67 and 1.50, therefore meeting the predefined immunological equivalence criteria. For the seroconversion rate of poliovirus types 1, 2 and 3, the pooled sIPV group was non-inferior to the IPV group. The incidence of solicited and unsolicited adverse reactions (ARs) was similar in the pooled sIPV lots and the IPV group, and most of them were mild to moderate in severity. Non-vaccine-related serious adverse events (SAEs) were reported.
Conclusions: Three consecutive lots of sIPV demonstrated robust and consistent immunogenicity. The safety and tolerability of the sIPV was acceptable and similar to that of the IPV.
Keywords: Sabin strain; immunogenicity; inactivated poliovirus vaccine; lot-to-lot consistency; safety.
Conflict of interest statement
T.Z., W.H., J.W. and L.X. are employees of Sinovac Biotech Co., Ltd. All the other authors declare no conflicts of interest.
Figures
Similar articles
-
Safety, immunogenicity, and lot-to-lot consistency of a multidose Sabin strain-based inactivated polio vaccine: a phase III, randomized, blinded, positive-control clinical trial in infants aged 2 months.Int J Infect Dis. 2023 May;130:20-27. doi: 10.1016/j.ijid.2023.01.020. Epub 2023 Jan 20. Int J Infect Dis. 2023. PMID: 36682682 Clinical Trial.
-
Safety and immunogenicity of 3 formulations of a Sabin inactivated poliovirus vaccine produced on the PER.C6® cell line: A phase 2, double-blind, randomized, controlled study in infants vaccinated at 6, 10 and 14 weeks of age.Hum Vaccin Immunother. 2022 Nov 30;18(5):2044255. doi: 10.1080/21645515.2022.2044255. Epub 2022 Mar 28. Hum Vaccin Immunother. 2022. PMID: 35344464 Free PMC article. Clinical Trial.
-
Immunogenicity of three sequential schedules with Sabin inactivated poliovirus vaccine and bivalent oral poliovirus vaccine in Zhejiang, China: an open-label, randomised, controlled trial.Lancet Infect Dis. 2020 Sep;20(9):1071-1079. doi: 10.1016/S1473-3099(19)30738-8. Epub 2020 May 19. Lancet Infect Dis. 2020. PMID: 32442523 Clinical Trial.
-
Safety and immunogenicity of experimental stand-alone trivalent, inactivated Sabin-strain polio vaccine formulations in healthy infants: A randomized, observer-blind, controlled phase 1/2 trial.Vaccine. 2020 Jul 14;38(33):5313-5323. doi: 10.1016/j.vaccine.2020.05.081. Epub 2020 Jun 17. Vaccine. 2020. PMID: 32563609 Free PMC article. Clinical Trial.
-
Lot-to-Lot Variation.Clin Biochem Rev. 2018 May;39(2):51-60. Clin Biochem Rev. 2018. PMID: 30473592 Free PMC article. Review.
Cited by
-
Observational analysis of the immunogenicity and safety of various types of spinal muscular atrophy vaccines.Inflammopharmacology. 2024 Apr;32(2):1025-1038. doi: 10.1007/s10787-023-01395-7. Epub 2024 Feb 3. Inflammopharmacology. 2024. PMID: 38308795 Clinical Trial.
-
Sustained Immune Persistence Five Years Post-Completion of Four-Dose sIPV Vaccination.Vaccines (Basel). 2025 Feb 27;13(3):253. doi: 10.3390/vaccines13030253. Vaccines (Basel). 2025. PMID: 40266111 Free PMC article.
References
-
- Dixon B. Medicine and the media: Polio still paralyses (Albert Sabin, Jonas Salk) Br. J. Hosp. Med. 1977;18:595. - PubMed
-
- World Health Organization Does Polio Still Exist? Is It Curable? [(accessed on 17 November 2021)]. Available online: http://www.who.int/features/qa/07/en/
-
- Centers for Disease Control and Prevention What We Do. [(accessed on 3 January 2022)]; Available online: https://www.cdc.gov/polio/what-we-do/
-
- Centers for Disease Control and Prevention Certification of poliomyelitis eradication—Western Pacific Region, October 2000. MMWR Morb. Mortal. Wkly Rep. 2001;50:1–3. - PubMed
-
- International Graduate Programs for Educators Polio This Week. [(accessed on 17 November 2021)]. Available online: https://polioeradication.org/polio-today/polio-now/this-week/
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials