Humoral and Cellular Responses to COVID-19 Vaccination Indicate the Need for Post-Vaccination Testing in Frail Population
- PMID: 35214717
- PMCID: PMC8875521
- DOI: 10.3390/vaccines10020260
Humoral and Cellular Responses to COVID-19 Vaccination Indicate the Need for Post-Vaccination Testing in Frail Population
Abstract
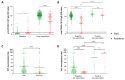
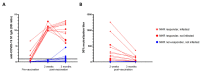
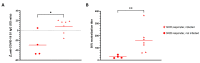
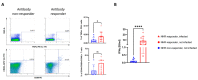
Despite the high efficacy of the BNT162b2 vaccine in the general population, data on its immunogenicity among frail elderly individuals are limited. Recently, levels of anti-SARS-CoV-2 spike IgG antibodies and serum neutralization titers were confirmed as good immune markers of protection against the virus, with evidence showing a reverse correlation between these two parameters and susceptibility to infection. Here we analyzed sera from 138 nursing home residents (median age of 88.9 years) and 312 nursing home staff (median age of 50.7 years) to determine the humoral response to two doses of the BNT162b2 vaccine, and found markedly decreased serum anti-spike antibody levels and neutralization titers in the nursing home resident (NHR) group, with over 11% non-responders compared to only 1.3% among the controls. Moreover, three months post-vaccination, a significant decrease in antibody titers was observed in COVID-19-naive nursing home residents. Subsequent flow cytometry and interferon gamma secretion analyses indicated that antibody non-responders among NHRs also failed to mount cellular responses. The presented data emphasize that additional measures are needed in the population of frail elderly individuals. Given the high proportion of non-responders among NHRs, continued monitoring should be considered in this group.
Keywords: SARS-CoV-2; antibodies; cellular immunity; nursing home; vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Voysey M., Clemens C.S.A., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie L.V., Barnabas S.L., Bhorat Q.E., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

