Optimization of Heat-Resistance Technology for a Duck Hepatitis Lyophilized Live Vaccine
- PMID: 35214727
- PMCID: PMC8880185
- DOI: 10.3390/vaccines10020269
Optimization of Heat-Resistance Technology for a Duck Hepatitis Lyophilized Live Vaccine
Abstract
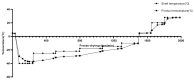
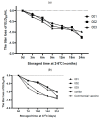
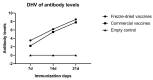
In this study, to improve the quality of a live attenuated vaccine for duck viral hepatitis (DHV), the lyophilization of a heat-resistant duck hepatitis virus vaccine was optimized. The optimized heat protectors were made of 10% sucrose, 1.2% pullulan, 0.5% PVP, and 1% arginine, etc., with a titer freeze-drying loss of ≤0.50 Lg. The vaccine product's valence measurements demonstrated the following: the vaccine could be stored at 2-8 °C for 18 months with a virus titer loss ≤0.91 Lg; at 37 °C for 10 days with a virus valence loss ≤0.89 Lg; and at 45 °C for 3 days with a virus titer loss ≤0.90 Lg. Regarding safety, no deaths occurred in two-day-old ducklings immunized with a 10 times dose vaccine; their energy, diet, and weight gain were all normal, demonstrating that the DHV heat-resistant vaccines were safe for ducklings and did not cause any immune side effects. Duck viral hepatitis freeze-dried vaccine began to produce antibodies at 7 d after immunization, reached above 5.0 on 14 d, and reached above 7.0 on 21 d, showing a continuous upward trend. This indicates that duck viral hepatitis vaccine has a good immunogen level. The optimization of the freeze-drying process saves costs and also improves the quality of the freeze-drying products, which provides important theoretical and technical support for the further study of vaccine products.
Keywords: DHV; optimization of the freeze-drying process; stabilizers; thermostability.
Conflict of interest statement
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Screening and Stability Evaluation of Freeze-Dried Protective Agents for a Live Recombinant Pseudorabies Virus Vaccine.Vaccines (Basel). 2024 Jan 9;12(1):65. doi: 10.3390/vaccines12010065. Vaccines (Basel). 2024. PMID: 38250878 Free PMC article.
-
Development of a stabilizer for lyophilization of an attenuated duck viral hepatitis vaccine.Poult Sci. 2010 Jun;89(6):1167-70. doi: 10.3382/ps.2009-00620. Poult Sci. 2010. PMID: 20460663
-
Live vaccine preserved at room temperature: Preparation and characterization of a freeze-dried classical swine fever virus vaccine.Vaccine. 2020 Dec 14;38(52):8371-8378. doi: 10.1016/j.vaccine.2020.10.093. Epub 2020 Nov 13. Vaccine. 2020. PMID: 33199076
-
Stability of yellow fever vaccine.Dev Biol Stand. 1996;87:219-25. Dev Biol Stand. 1996. PMID: 8854020 Review.
-
Freeze-drying of live virus vaccines: A review.Vaccine. 2015 Oct 13;33(42):5507-5519. doi: 10.1016/j.vaccine.2015.08.085. Epub 2015 Sep 26. Vaccine. 2015. PMID: 26364685 Review.
Cited by
-
Freeze-drying for the preservation of immunoengineering products.iScience. 2022 Sep 13;25(10):105127. doi: 10.1016/j.isci.2022.105127. eCollection 2022 Oct 21. iScience. 2022. PMID: 36267916 Free PMC article. Review.
-
Trehalose-loaded LNPs enhance mRNA stability and bridge in vitro in vivo efficacy gap.NPJ Vaccines. 2025 Aug 20;10(1):201. doi: 10.1038/s41541-025-01253-3. NPJ Vaccines. 2025. PMID: 40835843 Free PMC article.
-
Lyophilization process optimization and molecular dynamics simulation of mRNA-LNPs for SARS-CoV-2 vaccine.NPJ Vaccines. 2023 Oct 9;8(1):153. doi: 10.1038/s41541-023-00732-9. NPJ Vaccines. 2023. PMID: 37813912 Free PMC article.
-
Screening and Stability Evaluation of Freeze-Dried Protective Agents for a Live Recombinant Pseudorabies Virus Vaccine.Vaccines (Basel). 2024 Jan 9;12(1):65. doi: 10.3390/vaccines12010065. Vaccines (Basel). 2024. PMID: 38250878 Free PMC article.
-
Thermostable vacuum foam dried Newcastle disease vaccine: Process optimization and pilot-scale study.Appl Microbiol Biotechnol. 2024 Jun 5;108(1):359. doi: 10.1007/s00253-024-13174-7. Appl Microbiol Biotechnol. 2024. PMID: 38836885 Free PMC article.
References
-
- Song S., Li P., Zhang R., Chen J., Lan J., Lin S., Guo G., Xie Z., Jiang S. Oral vaccine of recombinant Lactococcus lactis expressing the VP1 protein of duck hepatitis A virus type 3 induces mucosal and systemic immune responses. Vaccine. 2019;37:4364–4369. doi: 10.1016/j.vaccine.2019.06.026. - DOI - PubMed
-
- Levine P., Hofstod M.P. Duck disease investigation. Annu. Rep. N. Y. State Vet. Coll. Ithaca. 1945:55–56.
LinkOut - more resources
Full Text Sources

