A Lateral Flow Immunoassay Coupled with a Spectrum-Based Reader for SARS-CoV-2 Neutralizing Antibody Detection
- PMID: 35214731
- PMCID: PMC8877288
- DOI: 10.3390/vaccines10020271
A Lateral Flow Immunoassay Coupled with a Spectrum-Based Reader for SARS-CoV-2 Neutralizing Antibody Detection
Abstract
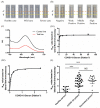
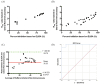
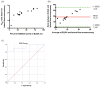
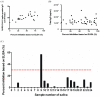
As of August 2021, there have been over 200 million confirmed case of coronavirus disease 2019 caused by severe acute respiratory syndrome coronavirus and more than 4 million COVID-19-related deaths globally. Although real-time polymerase chain reaction is considered to be the primary method of detection for SARS-CoV-2 infection, the use of serological assays for detecting COVID-19 antibodies has been shown to be effective in aiding with diagnosis, particularly in patients who have recovered from the disease and those in later stages of infection. Since it has a high detection rate and few limitations compared to conventional enzyme-linked immunosorbent assay protocols, we used a lateral flow immunoassay as our diagnostic tool of choice. Since lateral flow immunoassay results interpreted by the naked eye may lead to erroneous diagnoses, we developed an innovative, portable device with the capacity to capture a high-resolution reflectance spectrum as a means of promoting diagnostic accuracy. We combined this spectrum-based device with commercial lateral flow immunoassays to detect the neutralizing antibody in serum samples collected from 30 COVID-19-infected patients (26 mild cases and four severe cases). The results of our approach, lateral flow immunoassays coupled with a spectrum-based reader, demonstrated a 0.989 area under the ROC curve, 100% sensitivity, 95.7% positive predictive value, 87.5% specificity, and 100% negative predictive value. As a result, our approach exhibited great value for neutralizing antibody detection. In addition to the above tests, we also tested plasma samples from 16 AstraZeneca-vaccinated (ChAdOx1nCoV-19) patients and compared our approach and enzyme-linked immunosorbent assay results to see whether our approach could be applied to vaccinated patients. The results showed a high correlation between these two approaches, indicating that the lateral flow immunoassay coupled with a spectrum-based reader is a feasible approach for diagnosing the presence of a neutralizing antibody in both COVID-19-infected and vaccinated patients.
Keywords: AstraZeneca; coronavirus disease 2019 (COVID-19); lateral flow immunoassay; neutralizing antibody; severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- WHO WHO Statement Regarding Cluster of Pneumonia Cases in Wuhan, China. [(accessed on 7 January 2022)]. Available online: https://www.who.int/china/news/detail/09-01-2020-who-statement-regarding....
-
- WHO Coronavirus (COVID-19) Dashboard. [(accessed on 18 April 2021)]. Available online: https://covid19.who.int/
-
- Suthar M.S., Zimmerman M.G., Kauffman R.C., Mantus G., Linderman S.L., Hudson W.H., Vanderheiden A., Nyhoff L., Davis C.W., Adekunle O., et al. Rapid Generation of Neutralizing Antibody Responses in COVID-19 Patients. Cell Rep. Med. 2020;1:100040. doi: 10.1016/j.xcrm.2020.100040. - DOI - PMC - PubMed
-
- Wu F., Wang A., Liu M., Wang Q., Chen J., Xia S., Ling Y., Zhang Y., Xun J., Lu L., et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv. 2020 doi: 10.2139/ssrn.3566211. preprint . - DOI
-
- Wang X., Guo X., Xin Q., Pan Y., Hu Y., Li J., Chu Y., Feng Y., Wang Q. Neutralizing Antibody Responses to Severe Acute Respiratory Syndrome Coronavirus 2 in Coronavirus Disease 2019 Inpatients and Convalescent Patients. Clin. Infect. Dis. 2020;71:2688–2694. doi: 10.1093/cid/ciaa721. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

