Response to Vaccines in Patients with Immune-Mediated Inflammatory Diseases: A Narrative Review
- PMID: 35214755
- PMCID: PMC8877652
- DOI: 10.3390/vaccines10020297
Response to Vaccines in Patients with Immune-Mediated Inflammatory Diseases: A Narrative Review
Abstract
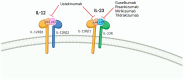
Patients with immune-mediated inflammatory diseases (IMIDs), such as rheumatoid arthritis and inflammatory bowel disease, are at increased risk of infection. International guidelines recommend vaccination to limit this risk of infection, although live attenuated vaccines are contraindicated once immunosuppressive therapy has begun. Biologic therapies used to treat IMIDs target the immune system to stop chronic pathogenic process but may also attenuate the protective immune response to vaccines. Here, we review the current knowledge regarding vaccine responses in IMID patients receiving treatment with biologic therapies, with a focus on the interleukin (IL)-12/23 inhibitors. B cell-depleting therapies, such as rituximab, strongly impair vaccines immunogenicity, and tumor necrosis factor (TNF) inhibitors and the cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) fusion protein abatacept are also associated with attenuated antibody responses, which are further diminished in patients taking concomitant immunosuppressants. On the other hand, integrin, IL-6, IL-12/23, IL-17, and B-cell activating factor (BAFF) inhibitors do not appear to affect the immune response to several vaccines evaluated. Importantly, treatment with biologic therapies in IMID patients is not associated with an increased risk of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or developing severe disease. However, the efficacy of SARS-CoV-2 vaccines on IMID patients may be reduced compared with healthy individuals. The impact of biologic therapies on the response to SARS-CoV-2 vaccines seems to replicate what has been described for other vaccines. SARS-CoV-2 vaccination appears to be safe and well tolerated in IMID patients. Attenuated but, in general, still protective responses to SARS-CoV-2 vaccination in the context of certain therapies warrant current recommendations for a third primary dose in IMID patients treated with immunosuppressive drugs.
Keywords: immune response; immune-mediated inflammatory diseases; interleukins; vaccine.
Conflict of interest statement
J.R.R. declares no conflicts of interest/financial interests. M.S. has served as a speaker and an advisory board member for Janssen, Gilead, Pfizer, MSD, Angelini, Astellas Ph., and Shionogi and has received research funding from ViiV/GSK. B.G. and S.D.-C. are employees of Janssen.
Figures
References
-
- Wisniewski A., Kirchgesner J., Seksik P., Landman C., Bourrier A., Nion-Larmurier I., Marteau P., Cosnes J., Sokol H., Beaugerie L., et al. Increased incidence of systemic serious viral infections in patients with inflammatory bowel disease associates with active disease and use of thiopurines. United Eur. Gastroenterol. J. 2020;8:303–313. doi: 10.1177/2050640619889763. - DOI - PMC - PubMed
-
- Yiu Z.Z.N., Sorbe C., Lunt M., Rustenbach S.J., Kuhl L., Augustin M., Mason K.J., Ashcroft D.M., Griffiths C.E.M., Warren R.B., et al. Development and validation of a multivariable risk prediction model for serious infection in patients with psoriasis receiving systemic therapy. Br. J. Dermatol. 2019;180:894–901. doi: 10.1111/bjd.17421. - DOI - PMC - PubMed
-
- Singh J.A., Guyatt G., Ogdie A., Gladman D.D., Deal C., Deodhar A., Dubreuil M., Dunham J., Husni M.E., Kenny S., et al. 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Care Res. 2019;71:2–29. doi: 10.1002/acr.23789. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous