New-Onset Kidney Diseases after COVID-19 Vaccination: A Case Series
- PMID: 35214760
- PMCID: PMC8880359
- DOI: 10.3390/vaccines10020302
New-Onset Kidney Diseases after COVID-19 Vaccination: A Case Series
Abstract
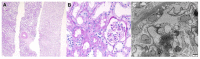
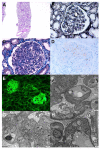
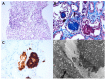
Various vaccines against COVID-19 have been developed and proven to be effective, but their side effects, especially on kidney function, are not yet known in detail. In this study, we report the clinical courses and histopathologic findings of new-onset kidney diseases after COVID-19 vaccination as confirmed via kidney biopsy. Five patients aged 42 to 77 years were included in this study, and baseline kidney function was normal in all patients. The biopsy-proven diagnosis indicated newly developed kidney diseases: (1) IgA nephropathy presenting with painless gross hematuria, (2) minimal change disease presenting with nephrotic syndrome, (3) thrombotic microangiopathy, and (4) two cases of acute tubulointerstitial nephritis presenting with acute kidney injury. Individualized treatment was applied as per disease severity and underlying pathology, and the treatment outcomes of all patients were improved. Since this is not a controlled study, the specific pathophysiologic link and causality between the incidence of kidney diseases and COVID-19 vaccination are difficult to confirm. However, clinicians need to consider the possibility that kidney diseases may be provoked by vaccines in patients who have renal symptoms.
Keywords: COVID-19; IgA nephropathy; kidney biopsy; kidney disease; minimal change disease; thrombotic microangiopathy; tubulointerstitial nephritis; vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

