An Observational Study to Assess the Effectiveness of 4CMenB against Meningococcal Disease and Carriage and Gonorrhea in Adolescents in the Northern Territory, Australia-Study Protocol
- PMID: 35214767
- PMCID: PMC8880162
- DOI: 10.3390/vaccines10020309
An Observational Study to Assess the Effectiveness of 4CMenB against Meningococcal Disease and Carriage and Gonorrhea in Adolescents in the Northern Territory, Australia-Study Protocol
Abstract
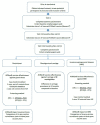
Invasive meningococcal disease (IMD) causes significant morbidity and mortality worldwide with serogroup B being the predominant serogroup in Australia and other countries for the past few decades. The licensed 4CMenB vaccine is effective in preventing meningococcal B disease. Emerging evidence suggests that although 4CMenB impact on carriage is limited, it may be effective against gonorrhoea due to genetic similarities between Neisseria meningitidis and Neisseria gonorrhoeae. This study protocol describes an observational study that will assess the effect of the 4CMenB vaccine against meningococcal carriage, IMD and gonorrhoea among adolescents in the Northern Territory (NT). All 14-19-year-olds residing in the NT with no contraindication for 4CMenB vaccine will be eligible to participate in this cohort study. Following consent, two doses of 4CMenB vaccine will be administered two months apart. An oropharyngeal swab will be collected at baseline and 12 months to detect pharyngeal carriage of Neisseria meningitidis by PCR. The main methodological approaches to assess the effect of 4CMenB involve a nested case control analysis and screening method to assess vaccine effectiveness and an Interrupted Time Series regression analysis to assess vaccine impact. Research ethics approvals have been obtained from Menzies and Central Australian Human Research Ethics Committees and the Western Australian Aboriginal Health Ethics Committee. Results will be provided in culturally appropriate formats for NT remote and regional communities and published in international peer reviewed journals. ClinicalTrials.gov Identifier: NCT04398849.
Keywords: 4CMenB; gonorrhoea; meningitis; meningococcal disease; vaccination; vaccine.
Conflict of interest statement
H.S.M. is an investigator on clinical trials of investigational vaccines sponsored by Industry. H.S.M.’s, institution receives funding from Industry (GSK, Pfizer, Sanofi-Pasteur, Novavax) for Investigator led research and for sponsored studies. H.S.M. receives no personal payments from Industry. P.R. has served on scientific advisory boards for meningococcal vaccines for GlaxoSmithKline and Pfizer on behalf of the University of Western Australia. His Institution has received funding for investigator grants outside the submitted work from GlaxoSmithKline. His institution has received funding for industry sponsored multicentre studies for GlaxoSmithKline, Pfizer outside the submitted work. He has not received personal remuneration for this work. Other authors report no conflict of interest.
References
-
- Borrow R., Alarcon P., Carlos J., Caugant D.A., Christensen H., Debbag R., De Wals P., Echaniz-Aviles G., Findlow J., Head C., et al. The Global Meningococcal Initiative: Global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev. Vaccines. 2017;16:313–328. doi: 10.1080/14760584.2017.1258308. - DOI - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous