Clostridial Diseases of Horses: A Review
- PMID: 35214776
- PMCID: PMC8876495
- DOI: 10.3390/vaccines10020318
Clostridial Diseases of Horses: A Review
Abstract
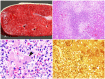
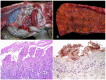
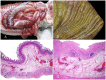
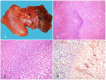
The clostridial diseases of horses can be divided into three major groups: enteric/enterotoxic, histotoxic, and neurotoxic. The main enteric/enterotoxic diseases include those produced by Clostridium perfringens type C and Clostridioides difficile, both of which are characterized by enterocolitis. The main histotoxic diseases are gas gangrene, Tyzzer disease, and infectious necrotic hepatitis. Gas gangrene is produced by one or more of the following microorganisms: C. perfringens type A, Clostridium septicum, Paeniclostridium sordellii, and Clostridium novyi type A, and it is characterized by necrotizing cellulitis and/or myositis. Tyzzer disease is produced by Clostridium piliforme and is mainly characterized by multifocal necrotizing hepatitis. Infectious necrotic hepatitis is produced by Clostridium novyi type B and is characterized by focal necrotizing hepatitis. The main neurotoxic clostridial diseases are tetanus and botulism, which are produced by Clostridium tetani and Clostridium botulinum, respectively. Tetanus is characterized by spastic paralysis and botulism by flaccid paralysis. Neither disease present with specific gross or microscopic lesions. The pathogenesis of clostridial diseases involves the production of toxins. Confirming a diagnosis of some of the clostridial diseases of horses is sometimes difficult, mainly because some agents can be present in tissues of normal animals. This paper reviews the main clostridial diseases of horses.
Keywords: Tyzzer disease; botulism; clostridial diseases; colitis; enteritis; enterocolitis; gas gangrene; horses; infectious necrotic hepatitis; review; tetanus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Prescott J.F., MacInnes J.I., Wu A.K.K. Clostridial Diseases of Animals. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2016. Taxonomic Relationships among the Clostridia; pp. 1–5.
-
- Rood J.I. Clostridial Diseases of Animals. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2016. General Physiological and Virulence Properties of the Pathogenic Clostridia; pp. 7–12.
-
- Fernandez-Miyakawa M.E., Fisher D.J., Poon R., Sayeed S., Adams V., Rood J.I., McClane B.A., Uzal F.A. Both Epsilon-Toxin and Beta-Toxin Are Important for the Lethal Properties of Clostridium Perfringens Type B Isolates in the Mouse Intravenous Injection Model. Infect. Immun. 2007;75:1443–1452. doi: 10.1128/IAI.01672-06. - DOI - PMC - PubMed

