Higher SARS-CoV-2 Spike Binding Antibody Levels and Neutralization Capacity 6 Months after Heterologous Vaccination with AZD1222 and BNT162b2
- PMID: 35214780
- PMCID: PMC8880180
- DOI: 10.3390/vaccines10020322
Higher SARS-CoV-2 Spike Binding Antibody Levels and Neutralization Capacity 6 Months after Heterologous Vaccination with AZD1222 and BNT162b2
Abstract
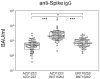
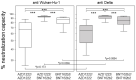
Within a year after the emergence of SARS-CoV-2, several vaccines had been developed, clinically evaluated, proven to be efficacious in preventing symptomatic disease, and licensed for global use. The remaining questions about the vaccines concern the duration of protection offered by vaccination and its efficacy against variants of concern. Therefore, we set out to analyze the humoral and cellular immune responses 6 months into homologous and heterologous prime-boost vaccinations. We recruited 190 health care workers and measured their anti-spike IgG levels, their neutralizing capacities against the Wuhan-Hu-1 strain and the Delta variant using a surrogate viral neutralization test, and their IFNγ-responses towards SARS-CoV-2-derived spike peptides. We here show that IFNγ secretion in response to peptide stimulation was significantly enhanced in all three vaccination groups and comparable in magnitude. In contrast, the heterologous prime-boost regimen using AZD1222 and BNT162b2 yielded the highest anti-spike IgG levels, which were 3-4.5 times more than the levels resulting from homologous AZD1222 and BNT162b2 vaccination, respectively. Likewise, the neutralizing capacity against both the wild type as well as the Delta receptor binding domains was significantly higher following the heterologous prime-boost regimen. In conclusion, our results suggest that mixing different SARS-CoV-2 vaccines might lead to more efficacious and longer-lasting humoral protection against breakthrough infections.
Keywords: COVID-19; SARS-CoV-2; heterologous prime-boost vaccination; humoral and cellular immune response; variant of concern.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Rahman M.M., Bhattacharjee B., Farhana Z., Hamiduzzaman M., Chowdhury M.A.B., Hossain M.S., Siddiqee M.H., Islam M.Z., Raheem E., Uddin M.J. Age-related risk factors and severity of SARS-CoV-2 infection: A systematic review and meta-analysis. J. Prev. Med. Hyg. 2021;62:E329–E371. doi: 10.15167/2421-4248/jpmh2021.62.2.1946. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

