Comparative Immunogenicity of COVID-19 Vaccines in a Population-Based Cohort Study with SARS-CoV-2-Infected and Uninfected Participants
- PMID: 35214782
- PMCID: PMC8875516
- DOI: 10.3390/vaccines10020324
Comparative Immunogenicity of COVID-19 Vaccines in a Population-Based Cohort Study with SARS-CoV-2-Infected and Uninfected Participants
Abstract
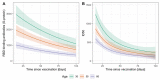
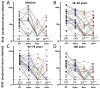
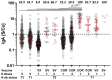
To assess vaccine immunogenicity in non-infected and previously infected individuals in a real-world scenario, SARS-CoV-2 antibody responses were determined during follow-up 2 (April 2021) of the population-based Tirschenreuth COVID-19 cohort study comprising 3378 inhabitants of the Tirschenreuth county aged 14 years or older. Seronegative participants vaccinated once with Vaxzevria, Comirnaty, or Spikevax had median neutralizing antibody titers ranging from ID50 = 25 to 75. Individuals with two immunizations with Comirnaty or Spikevax had higher median ID50s (of 253 and 554, respectively). Regression analysis indicated that both increased age and increased time since vaccination independently decreased RBD binding and neutralizing antibody levels. Unvaccinated participants with detectable N-antibodies at baseline (June 2020) revealed a median ID50 of 72 at the April 2021 follow-up. Previously infected participants that received one dose of Vaxzevria or Comirnaty had median ID50 to 929 and 2502, respectively. Individuals with a second dose of Comirnaty given in a three-week interval after the first dose did not have higher median antibody levels than individuals with one dose. Prior infection also primed for high systemic IgA levels in response to one dose of Comirnaty that exceeded IgA levels observed after two doses of Comirnaty in previously uninfected participants. Neutralizing antibody levels targeting the spike protein of Beta and Delta variants were diminished compared to the wild type in vaccinated and infected participants.
Keywords: Comirnaty; SARS-CoV-2; Spikevax; Vaxzevria; immunogenicity; neutralizing antibodies; population-based cohort; vaccination; variants of concern VoC.
Conflict of interest statement
The authors declare that no competing interest or conflict of interest exist. The authors have no financial or proprietary interest in any material discussed in this article.
Figures


References
-
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

