SARS-CoV-2 mRNA Vaccination in People with Multiple Sclerosis Treated with Fingolimod: Protective Humoral Immune Responses May Develop after the Preferred Third Shot
- PMID: 35214799
- PMCID: PMC8875864
- DOI: 10.3390/vaccines10020341
SARS-CoV-2 mRNA Vaccination in People with Multiple Sclerosis Treated with Fingolimod: Protective Humoral Immune Responses May Develop after the Preferred Third Shot
Abstract
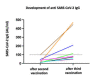
Evidence suggests limited development of protective IgG responses to mRNA-based vaccines in sphingosine-1-phosphate receptor (S1PR)-modulator treated individuals with multiple sclerosis (MS). We studied the extent of the humoral immune response after the preferred third mRNA SARS-CoV-2 vaccine in S1PR-modulator treated people with MS (pwMS) and insufficient IgG responses after the standard immunization scheme. Eight pwMS that were treated with fingolimod received a third homologous SARS-CoV-2 mRNA vaccine dose, either the Moderna's mRNA-1273 or Pfizer-BioNTech's BNT162b2 vaccine. We quantified the serum levels of IgG antibodies against the receptor-binding domain of SARS-CoV-2 four weeks later. An antibody titer of 100 AU/mL or more was considered protective. After the third vaccination, we found clinically relevant IgG titers in four out of eight individuals (50%). We conclude that the humoral immune response may reach protective levels after the third preferred dose of the homologous SARS-CoV-2 mRNA vaccine. Vaccine shots in S1PR-modulator treated pwMS ahead of schedule may be a strategy to overcome insufficient humoral immune responses following the standard vaccination scheme.
Keywords: COVID-19; S1PR-modulator; SARS-CoV-2; fingolimod; humoral immune response; vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Sellner J., Jenkins T.M., von Oertzen T.J., Bassetti C.L., Beghi E., Bereczki D., Bodini D., Cavallieri F., Di Liberto G., Helbok R., et al. Primary prevention of COVID-19: Advocacy for vaccination from a neurological perspective. Eur. J. Neurol. 2021;28:3226–3229. doi: 10.1111/ene.14713. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous