Phytochemical Screening of Rosmarinus officinalis L. as a Potential Anticholinesterase and Antioxidant-Medicinal Plant for Cognitive Decline Disorders
- PMID: 35214846
- PMCID: PMC8877369
- DOI: 10.3390/plants11040514
Phytochemical Screening of Rosmarinus officinalis L. as a Potential Anticholinesterase and Antioxidant-Medicinal Plant for Cognitive Decline Disorders
Abstract
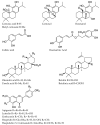
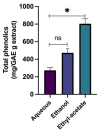
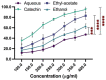
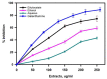
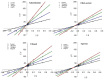
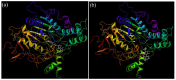
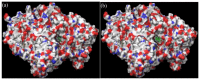
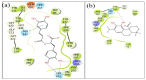
The inhibition of acetylcholinesterase (AChE) by cholinergic agents has been promoted as a potent strategy for treating and managing cognitive decline disorders. A wide range of natural products has long been used as potential sources or formulations of cholinergic inhibitors. Therefore, this study aimed to evaluate different Rosmarinus officinalis L. (R. officinalis) extracts for their AChE inhibitory activity using galanthamine as a standard AChE inhibitor. In this study, the ethyl-acetate extract (at a concentration of 250 µg/mL) exhibited the greatest inhibitory effect against AChE with significant inhibition of 75%, comparable to the inhibitor galanthamine with an inhibition of 88%. Kinetic analysis revealed that the extracts could induce a mixed type of inhibition, as observed in the case of galanthamine, with the highest increased Km and decreased Vmax values in the ethyl acetate extract. The antioxidant potential of the three extracts tested was found to be in the order of ethyl-acetate > ethanol > aqueous, with IC50 values of 272 µg/mL, 387 µg/mL, and 534 µg/mL, respectively. Ethyl-acetate was found to have the highest total phenolic content in all extracts. Further, in silico study showed structural binding characterization of rosmarinic acid and carnosic acid with human AChE enzyme. Rosmarinic acid showed strong binding and formed two hydrogen-bonding interactions with Ser-293 and Arg-296. In light of this, the ethyl-acetate extract of the plant may provide some novel potential pharmacological leads for treating and managing cognitive disorders such as Alzheimer's.
Keywords: Alzheimer’s disease; Rosmarinus officinalis; acetylcholinesterase; carnosic acid; molecular docking; rosemary; rosmarinic acid.
Conflict of interest statement
The authors declare no conflict of interest. This research did not involve any animal and/or human participant.
Figures
References
-
- NIH Alzheimer’s Disease Fact Sheet. [(accessed on 16 October 2021)];2021 July 8; Available online: https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet.
-
- Matthews K.A., Xu W., Gaglioti A.H., Holt J.B., Croft J.B., Mack D., McGuire L.C. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimers Dement. 2019;1:17–24. doi: 10.1016/j.jalz.2018.06.3063. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

