Jasmonate-Dependent Response of the Flower Abscission Zone Cells to Drought in Yellow Lupine
- PMID: 35214860
- PMCID: PMC8877524
- DOI: 10.3390/plants11040527
Jasmonate-Dependent Response of the Flower Abscission Zone Cells to Drought in Yellow Lupine
Abstract
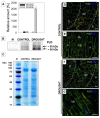
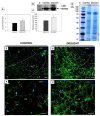
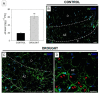
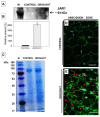
Lipid membranes, as primary places of the perception of environmental stimuli, are a source of various oxygenated polyunsaturated fatty acids-oxylipins-functioning as modulators of many signal transduction pathways, e.g., phytohormonal. Among exogenous factors acting on plant cells, special attention is given to drought, especially in highly sensitive crop species, such as yellow lupine. Here, we used this species to analyze the contribution of lipid-related enzymes and lipid-derived plant hormones in drought-evoked events taking place in a specialized group of cells-the flower abscission zone (AZ)-which is responsible for organ detachment from the plant body. We revealed that water deficits in the soil causes lipid peroxidation in these cells and the upregulation of phospholipase D, lipoxygenase, and, concomitantly, jasmonic acid (JA) strongly accumulates in AZ tissue. Furthermore, we followed key steps in JA conjugation and signaling under stressful conditions by monitoring the level and tissue localization of enzyme providing JA derivatives (JASMONATE RESISTANT1) and the JA receptor (CORONATINE INSENSITIVE1). Collectively, drought-triggered AZ activation during the process of flower abscission is closely associated with the lipid modifications, leading to the formation of JA, its conjugation, and induction of signaling pathways.
Keywords: CORONATINE INSENSITIVE1; JASMONATE RESISTANT1; abscission zone; drought; flower abscission; jasmonates; jasmonic acid; lipoxygenase; phospholipase D.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

