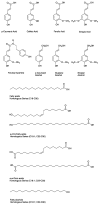
Figure 4
Overview of the regulation of suberin biosynthesis and assembly in wound-healing potato tubers. This overview offers a synthesis of findings, and proposes mechanisms of regulation at the levels of monomer biosynthesis, deposition, polymerization and assembly, based on the literature described in this review. Primary metabolic pathways (shaded green) yield precursors and energy molecules that feed into specialized suberin-related metabolic branches. For example, carbohydrate metabolism yields erythrose-4-phosphate and phospho-enol-pyruvate as precursors to the shikimate pathway and production of aromatic amino acids used as precursors for phenolic suberin biosynthesis. Pyruvate and glycolysis-derived glyceraldehyde-3-phosphate are used for the isoprenoid metabolism that yields the phytohormone abscisic acid (ABA) via the carotenoid pathway. Pyruvate is also a substrate for the tricarboxylic acid cycle that yields acetyl-CoA for fatty acid biosynthesis, and results in the generation of 16:0, 18:0 and 18:1 fatty acids that undergo various modifications for aliphatic suberin monomer production. Glycerol-3-phosphate is synthesized from the dehydrogenation of dihydroxyacetone phosphate produced during glycolysis. The biosynthesis of suberin poly(phenolic) domain monomers (dark red) may be regulated by WRKY and MYB TFs. WRKY1 regulates THT and 4CL in relation to phenylpropanoid metabolism in pathogen-infected aerial potato organs. MYB74 and MYB102 may also regulate phenylpropanoid metabolism in potato tuber suberization. ABA regulates the biosynthesis of several key suberin poly(aliphatic) domain monomers (blue) by positively impacting genes involved in their production. NAC103 acts as a transcriptional suppressor of fatty acid and aliphatic suberin-related genes, and is induced by ABA. MYB TFs such as MYB102 and MYB74 may positively regulate aliphatic suberin production. (See Table 1 for key suberin biosynthetic genes and Table 2 for other TFs that regulate phenylpropanoid and/or fatty acid biosynthesis in other species.) It is feasible that potato orthologs of TFs characterized in other species may regulate suberization in wounded tubers. Most phenolic monomers are solely polymerized and incorporated into the SPPD. Feruloyl-CoA can also be conjugated to very long-chain fatty acids (VLCFAs), ω-hydroxy and α,ω-dioic acids, and 1-alkanols to yield ferulate esters, including alkyl ferulates as SPAD-associated soluble wax components. Modified fatty acids can also be esterified to glycerol via the glycerol-3-phosphate acyltransferases (GPATs). Esterified aliphatic constituents are exported by ABCG1. These steps represent a point of convergence between the two major suberin biosynthetic pathways and are labeled as “convergent metabolism” (shaded purple) in the figure. MYB TFs such as MYB102 and MYB74 are putative regulators of the genes encoding convergent metabolic steps. The translocation of aliphatic monomers (alkanes, VLCFAs, modified fatty acids, 1-alkanols) that are not esterified to glycerol or feruloyl-CoA (i.e., soluble components destined for polymerization, or that remain non-polymerized as associated wax) has not been established in potato, but ABCG11 has predicted involvement. Phenolic monomers are thought to undergo a NADPH-dependent oxidase (NOX), superoxide dismutase (SOD), and anionic peroxidase (PRX)-mediated polymerization, whereas SPAD polymerization activities remain uncharacterized. CASP and GDSL-like proteins may play a role in SPAD polymerization. Phenolic suberin associated CASPs may recruit machinery such as NOX, SOD, PRX for polymerization, and influence enzymes involved in the organization of cell wall polysaccharides in a process that may be associated with SPPD deposition via localization of cell wall modifying activities. CASP1-like/CASP8 could regulate the linkage between two domains, their spatial organization, and/or the polymerization and deposition of esterified aliphatics that act as building blocks for SPAD assembly. CASP9 may coordinate aliphatic suberin assembly. GDSLs may act as “suberin synthases” at this stage of assembly, akin to cutin synthases. ABCG1 is required for the export of aliphatic suberin components. ABCG11 is suppressed by NAC103. ABCGs with varied substrate specificities and MYB TFs are likely involved in the export, deposition and polymerization of non-esterified aliphatics, as well as the organization of non-polymerized, soluble waxes. Cell wall modification machinery may be regulated by CASPs (e.g., CASP1B2-like/CASP9) to organize the deposition of polymerized aliphatics between the cell wall and plasma membrane, prior to secondary cell wall formation. Abbreviations: 4CL, 4-coumarate-CoA ligase; ABA, abscisic acid; ABCG1, ATP-binding cassette (ABC) subfamily G transporter 1; ABCG11, ATP-binding cassette (ABC) subfamily G transporter 11; ABCG6, ATP-binding cassette (ABC) subfamily G transporter 6; C3H, p-coumarate 3-hydroxylase; C3’H, p-coumaroyl quinate/shikimate 3’-hydroxylase; C4H, cinnamic acid 4-hydroxylase; CAD, cinnamyl alcohol dehydrogenase; CASP8, Casparian strip membrane domain protein 8; CASP9, Casparian strip membrane domain protein 9; CCoAOMT, caffeoyl-CoA O-methyltransferase; CCR, cinnamoyl CoA reductase; CER, ECERIFERUM; COMT, caffeic acid O-methyltransferase; CYP, cytochrome P450; CYP86A33, cytochrome P450 subfamily 86A 33; CYP86B12, cytochrome P450 subfamily 86B 12; CYTB5, cytochrome b5; F5H, ferulate 5-hydroxylase; FA, fatty acid; FAE, fatty acid elongase; GDSL, GDSL domain esterase/lipase; Glycerol-3P, glycerol-3-phosphate; GPAT5, glycerol-3-phosphate acyltransferase 5; GPAT6, glycerol-3-phosphate acyltransferase 6; HCAA, hydroxycinnamic acid amide; HCT, hydroxycinnamate transferase; KAR, β-ketoacyl-ACP reductase; KCS6, β-ketoacyl-CoA reductase 6; LACS, long-chain acyl-CoA synthetase; MYB, MYB family transcription factor; NAC, NAC domain transcription factor (NAM, no apical meristem, ATAF, Arabidopsis transcription activation factor, and CUC, cup-shaped cotyledon); NOX, NADPH-dependent oxidase; PAL, phenylalanine ammonia lyase; PRX, anionic peroxidase; SOD, superoxide dismutase; SPAD, suberin poly(aliphatic) domain; SPPD, suberin poly(phenolic) domain; THT, tyramine hydroxycinnamoyl transferase; VLCFA, very-long-chain fatty acid; WRKY, WRKY domain family transcription factor.