Adsorption and Fenton-like Degradation of Ciprofloxacin Using Corncob Biochar-Based Magnetic Iron-Copper Bimetallic Nanomaterial in Aqueous Solutions
- PMID: 35214908
- PMCID: PMC8880508
- DOI: 10.3390/nano12040579
Adsorption and Fenton-like Degradation of Ciprofloxacin Using Corncob Biochar-Based Magnetic Iron-Copper Bimetallic Nanomaterial in Aqueous Solutions
Abstract
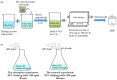
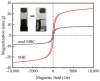
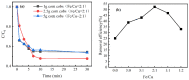
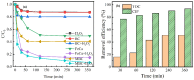
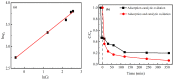
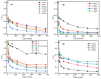
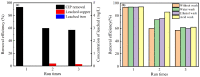
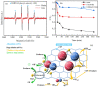
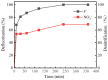
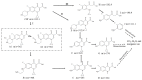
An economical corncob biochar-based magnetic iron-copper bimetallic nanomaterial (marked as MBC) was successfully synthesized and optimized through a co-precipitation and pyrolysis method. It was successfully used to activate H2O2 to remove ciprofloxacin (CIP) from aqueous solutions. This material had high catalytic activity and structural stability. Additionally, it had good magnetic properties, which can be easily separated from solutions. In MBC/H2O2, the removal efficiency of CIP was 93.6% within 360 min at optimal reaction conditions. The conversion of total organic carbon (TOC) reached 51.0% under the same situation. The desorption experiments concluded that adsorption and catalytic oxidation accounted for 34% and 66% on the removal efficiency of CIP, respectively. The influences of several reaction parameters were systematically evaluated on the catalytic activity of MBC. OH was proved to play a significant role in the removal of CIP through electron paramagnetic resonance (EPR) analysis and a free radical quenching experiment. Additionally, such outstanding removal efficiency can be attributed to the excellent electronic conductivity of MBC, as well as the redox cycle reaction between iron and copper ions, which achieved the continuous generation of hydroxyl radicals. Integrating HPLC-MS, ion chromatography and density functional theory (DFT) calculation results, and possible degradation of the pathways of the removal of CIP were also thoroughly discussed. These results provided a theoretical basis and technical support for the removal of CIP in water.
Keywords: AOPs; adsorption; advanced oxidation process; catalytic activity; ciprofloxacin; corncob biochar-based magnetic iron–copper bimetallic nanomaterial; fenton-like catalyst.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Removal of typical pollutant ciprofloxacin using iron-nitrogen co-doped modified corncob in the presence of hydrogen peroxide.RSC Adv. 2023 Nov 22;13(49):34335-34347. doi: 10.1039/d3ra06437a. eCollection 2023 Nov 22. RSC Adv. 2023. PMID: 38024979 Free PMC article.
-
Effects of activated carbon, biochar, and carbon nanotubes on the heterogeneous Fenton oxidation catalyzed by pyrite for ciprofloxacin degradation.Chemosphere. 2022 Dec;308(Pt 3):136427. doi: 10.1016/j.chemosphere.2022.136427. Epub 2022 Sep 16. Chemosphere. 2022. PMID: 36122753
-
Self-Fenton Cu-Mn catalysts for efficient ciprofloxacin removal: in-situ H2O2 generation and activation.J Environ Manage. 2025 Jun;384:125569. doi: 10.1016/j.jenvman.2025.125569. Epub 2025 Apr 28. J Environ Manage. 2025. PMID: 40300550
-
Catalytic Degradation of Ciprofloxacin in Aqueous Solution by Peroxymonosulfate Activated with a Magnetic CuFe2O4@Biochar Composite.Int J Mol Sci. 2023 Mar 16;24(6):5702. doi: 10.3390/ijms24065702. Int J Mol Sci. 2023. PMID: 36982776 Free PMC article.
-
Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes.J Hazard Mater. 2014 Jun 30;275:121-35. doi: 10.1016/j.jhazmat.2014.04.054. Epub 2014 May 2. J Hazard Mater. 2014. PMID: 24857896 Review.
Cited by
-
Reduced Graphene Oxide-Coated CuFeO2 with Fenton-like Catalytic Degradation Performance for Terramycin.Nanomaterials (Basel). 2022 Dec 9;12(24):4391. doi: 10.3390/nano12244391. Nanomaterials (Basel). 2022. PMID: 36558244 Free PMC article.
-
Copper(II) phosphate as a promising catalyst for the degradation of ciprofloxacin via photo-assisted Fenton-like process.Sci Rep. 2024 Mar 25;14(1):7007. doi: 10.1038/s41598-024-57542-9. Sci Rep. 2024. PMID: 38523152 Free PMC article.
-
Coactivation of Hydrogen Peroxide Using Pyrogenic Carbon and Magnetite for Sustainable Oxidation of Organic Pollutants.ACS Omega. 2024 Feb 1;9(6):6595-6605. doi: 10.1021/acsomega.3c07525. eCollection 2024 Feb 13. ACS Omega. 2024. PMID: 38371804 Free PMC article.
-
Enhancing catalyst stability: Immobilization of Cu-Fe catalyst in sodium alginate matrix for methyl orange removal via Fenton-like reaction.Heliyon. 2024 Jun 27;10(13):e33789. doi: 10.1016/j.heliyon.2024.e33789. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39040388 Free PMC article.
References
-
- Yu J., Tang L., Pang Y., Zeng G., Wang J., Deng Y., Liu Y., Feng H., Chen S., Ren X. Magnetic nitrogen-doped sludge-derived biochar catalysts for persulfate activation: Internal electron transfer mechanism. Chem. Eng. J. 2019;364:146–159. doi: 10.1016/j.cej.2019.01.163. - DOI
-
- Wang J., Tang L., Zeng G., Deng Y., Liu Y., Wang L., Zhou Y., Guo Z., Wang J., Zhang C. Atomic scale g-C3N4/Bi2WO6 2D/2D heterojunction with enhanced photocatalytic degradation of ibuprofen under visible light irradiation. Appl. Catal. B Environ. 2017;209:285–294. doi: 10.1016/j.apcatb.2017.03.019. - DOI
-
- Deng Y., Tang L., Zeng G., Zhu Z., Yan M., Zhou Y., Wang J., Liu Y., Wang J. Insight into highly efficient simultaneous photocatalytic removal of Cr(VI) and 2,4-diclorophenol under visible light irradiation by phosphorus doped porous ultrathin g-C3N4 nanosheets from aqueous media: Performance and reaction mechanism. Appl. Catal. B Environ. 2017;203:343–354. doi: 10.1016/j.apcatb.2016.10.046. - DOI
-
- Shah N.S., Ali Khan J., Sayed M., Ul Haq Khan Z., Sajid Ali H., Murtaza B., Khan H.M., Imran M., Muhammad N. Hydroxyl and sulfate radical mediated degradation of ciprofloxacin using nano zerovalent manganese catalyzed S2O82−. Chem. Eng. J. 2019;356:199–209. doi: 10.1016/j.cej.2018.09.009. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

