Formation of Aggregate-Free Gold Nanoparticles in the Cyclodextrin-Tetrachloroaurate System Follows Finke-Watzky Kinetics
- PMID: 35214912
- PMCID: PMC8875903
- DOI: 10.3390/nano12040583
Formation of Aggregate-Free Gold Nanoparticles in the Cyclodextrin-Tetrachloroaurate System Follows Finke-Watzky Kinetics
Abstract
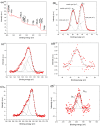
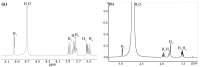
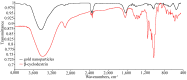
Cyclodextrin-capped gold nanoparticles are promising drug-delivery vehicles, but the technique of their preparation without trace amounts of aggregates is still lacking, and the size-manipulation possibility is very limited. In the present study, gold nanoparticles were synthesized by means of 0.1% (w/w) tetrachloroauric acid reduction with cyclodextrins at room temperature, at cyclodextrin concentrations of 0.001 M, 0.002 M and 0.004 M, and pH values of 11, 11.5 and 12. The synthesized nanoparticles were characterized by dynamic light scattering in both back-scattering and forward-scattering modes, spectrophotometry, X-ray photoelectron spectroscopy, transmission electron microscopy and Fourier-transform infrared spectroscopy. These techniques revealed 14.9% Au1+ on their surfaces. The Finke-Watzky kinetics of the reaction was demonstrated, but the actual growth mechanism turned out to be multistage. The synthesis kinetics and the resulting particle-size distribution were pH-dependent. The reaction and centrifugation conditions for the recovery of aggregate-free nanoparticles with different size distributions were determined. The absorbances of the best preparations were 7.6 for α-cyclodextrin, 8.9 for β-cyclodextrin and 7.5 for γ-cyclodextrin. Particle-size distribution by intensity was indicative of the complete absence of aggregates. The resulting preparations were ready to use without the need for concentration, filtration, or further purification. The synthesis meets the requirements of green chemistry.
Keywords: Finke–Watzky kinetics; cyclodextrin; drug delivery vehicle; gold nanoparticles; green chemistry; tetrachloroaurate reduction.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript.
Figures




References
-
- Dhiman P., Bhatia M. Pharmaceutical applications of cyclodextrins and their derivatives. J. Incl. Phenom. Macrocycl. Chem. 2020;98:171–186. doi: 10.1007/s10847-020-01029-3. - DOI
-
- Ochoa-Olmos O.E., León-Domínguez J.A., Contreras-Torres F.F., Sanchez-Nieto S., Basiuk E.V., Dinkova T.D. Transfor-mation of plant cell suspension cultures with amine-functionalized multi-walled carbon nanotubes. J. Nanosci. Nanotechnol. 2016;16:7461–7471. doi: 10.1166/jnn.2016.12815. - DOI
-
- Ma X., Quah B. Effects of surface charge on the fate and phytotoxicity of gold nanoparticles to Phaseolus vulgaris. J. Food Chem. Nanotechnol. 2016;2:57–65. doi: 10.17756/jfcn.2016-011. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

