Strategy for Conjugating Oligopeptides to Mesoporous Silica Nanoparticles Using Diazirine-Based Heterobifunctional Linkers
- PMID: 35214937
- PMCID: PMC8880541
- DOI: 10.3390/nano12040608
Strategy for Conjugating Oligopeptides to Mesoporous Silica Nanoparticles Using Diazirine-Based Heterobifunctional Linkers
Abstract
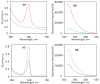
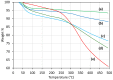
Successful strategies for the attachment of oligopeptides to mesoporous silica with pores large enough to load biomolecules should utilize the high surface area of pores to provide an accessible, protective environment. A two-step oligopeptide functionalization strategy is examined here using diazirine-based heterobifunctional linkers. Mesoporous silica nanoparticles (MSNPs) with average pore diameter of ~8 nm and surface area of ~730 m2/g were synthesized and amine-functionalized. Tetrapeptides Gly-Gly-Gly-Gly (GGGG) and Arg-Ser-Ser-Val (RSSV), and a peptide comprised of four copies of RSSV (4RSSV), were covalently attached via their N-terminus to the amine groups on the particle surface by a heterobifunctional linker, sulfo-succinimidyl 6-(4,4'-azipentanamido)hexanoate (sulfo-NHS-LC-diazirine, or SNLD). SNLD consists of an amine-reactive NHS ester group and UV-activable diazirine group, providing precise control over the sequence of attachment steps. Attachment efficiency of RSSV was measured using fluorescein isothiocyanate (FITC)-tagged RSSV (RSSV-FITC). TGA analysis shows similar efficiency (0.29, 0.31 and 0.26 mol peptide/mol amine, respectively) for 4G, RSSV and 4RSSV, suggesting a generalizable method of peptide conjugation. The technique developed here for the conjugation of peptides to MSNPs provides for their attachment in pores and can be translated to selective peptide-based separation and concentration of therapeutics from aqueous process and waste streams.
Keywords: conjugation; diazirine; heterobifunctional linker; mesoporous silica; nanoparticle; oligopeptide.
Conflict of interest statement
J.M.L. and D.T.R. are employees of Naprogenix Inc. and J.M.L. owns stock in the company. The funders of this work had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

